What Makes a Kinase Promiscuous for Inhibitors?
Hanson, S.M., Georghiou, G., Thakur, M.K., Miller, W.T., Rest, J.S., Chodera, J.D., Seeliger, M.A.(2019) Cell Chem Biol 26: 390-399.e5
- PubMed: 30612951
- DOI: https://doi.org/10.1016/j.chembiol.2018.11.005
- Primary Citation of Related Structures:
6BRJ, 6BSD - PubMed Abstract:
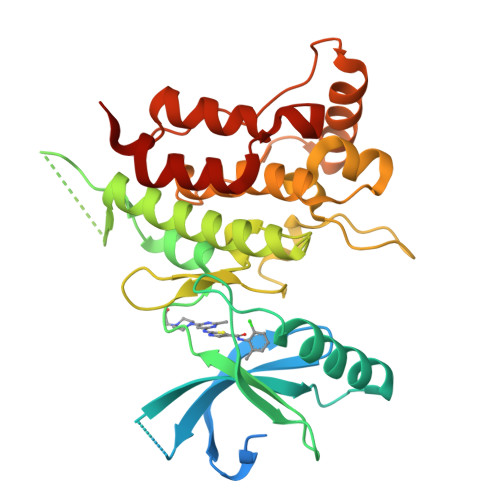
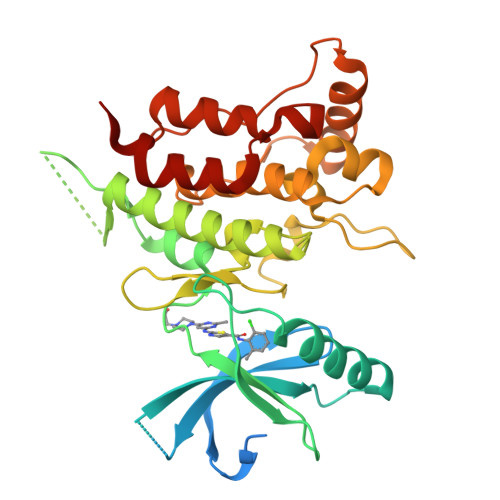
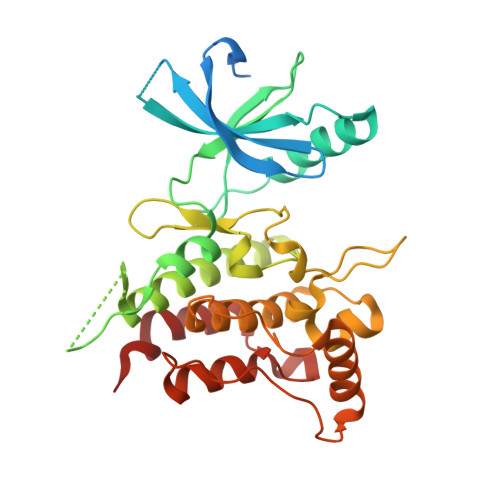
ATP-competitive kinase inhibitors often bind several kinases due to the high conservation of the ATP binding pocket. Through clustering analysis of a large kinome profiling dataset, we found a cluster of eight promiscuous kinases that on average bind more than five times more kinase inhibitors than the other 398 kinases in the dataset. To understand the structural basis of promiscuous inhibitor binding, we determined the co-crystal structure of the receptor tyrosine kinase DDR1 with the type I inhibitors dasatinib and VX-680. Surprisingly, we find that DDR1 binds these type I inhibitors in an inactive conformation typically reserved for type II inhibitors. Our computational and biochemical studies show that DDR1 is unusually stable in this inactive conformation, giving a mechanistic explanation for inhibitor promiscuity. This phenotypic clustering analysis provides a strategy to obtain functional insights not available by sequence comparison alone.
Organizational Affiliation:
Department of Pharmacological Sciences, Stony Brook University, Stony Brook, NY 11794-8651, USA; Computational and Systems Biology Program, Memorial Sloan Kettering Cancer Center, New York, NY 10065-1115, USA.