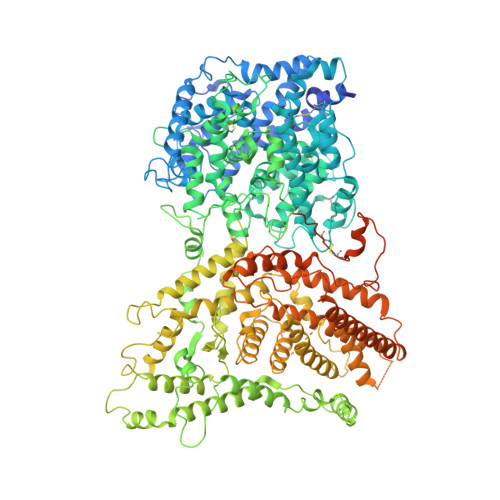
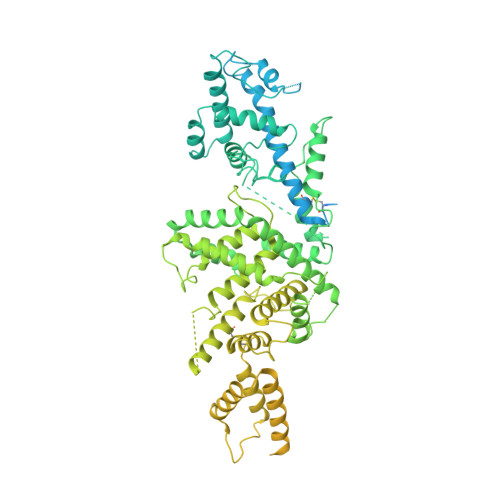
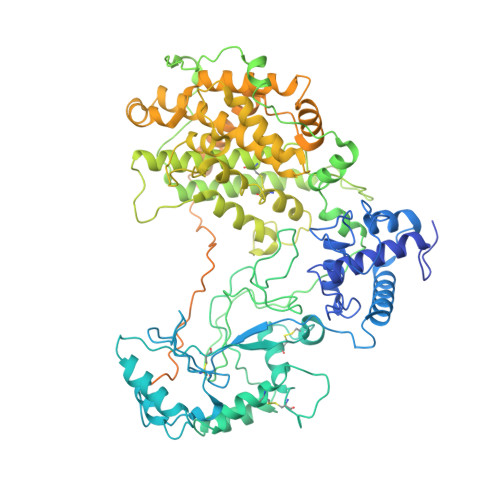
Malaria parasites use a soluble RhopH complex for erythrocyte invasion and an integral form for nutrient uptake.
Schureck, M.A., Darling, J.E., Merk, A., Shao, J., Daggupati, G., Srinivasan, P., Olinares, P.D.B., Rout, M.P., Chait, B.T., Wollenberg, K., Subramaniam, S., Desai, S.A.(2021) Elife 10
- PubMed: 33393463
- DOI: https://doi.org/10.7554/eLife.65282
- Primary Citation of Related Structures:
7KIY - PubMed Abstract:
Malaria parasites use the RhopH complex for erythrocyte invasion and channel-mediated nutrient uptake. As the member proteins are unique to Plasmodium spp., how they interact and traffic through subcellular sites to serve these essential functions is unknown. We show that RhopH is synthesized as a soluble complex of CLAG3, RhopH2, and RhopH3 with 1:1:1 stoichiometry. After transfer to a new host cell, the complex crosses a vacuolar membrane surrounding the intracellular parasite and becomes integral to the erythrocyte membrane through a PTEX translocon-dependent process. We present a 2.9 Å single-particle cryo-electron microscopy structure of the trafficking complex, revealing that CLAG3 interacts with the other subunits over large surface areas. This soluble complex is tightly assembled with extensive disulfide bonding and predicted transmembrane helices shielded. We propose a large protein complex stabilized for trafficking but poised for host membrane insertion through large-scale rearrangements, paralleling smaller two-state pore-forming proteins in other organisms.
Organizational Affiliation:
Laboratory of Malaria and Vector Research, NIAID, National Institutes of Health, Rockville, United States.