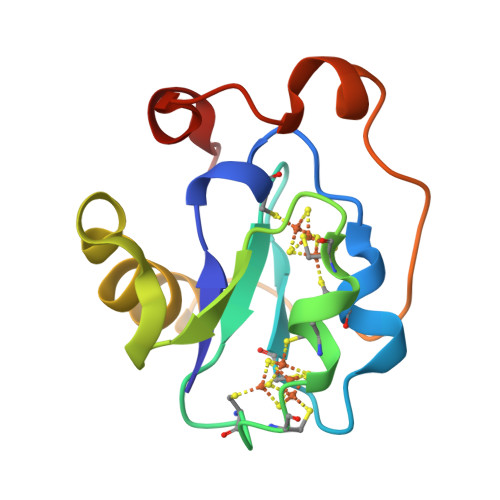
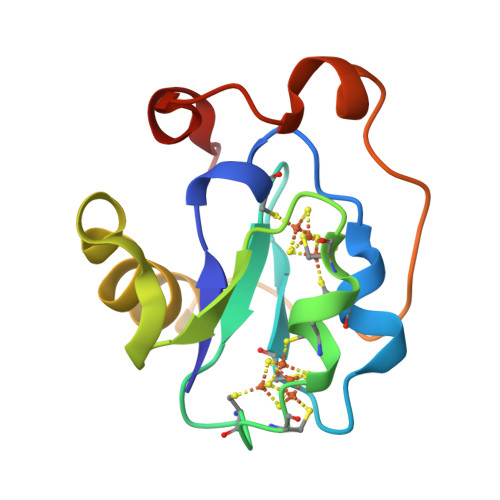
Site-directed mutagenesis of Azotobacter vinelandii ferredoxin I: [Fe-S] cluster-driven protein rearrangement.
Martin, A.E., Burgess, B.K., Stout, C.D., Cash, V.L., Dean, D.R., Jensen, G.M., Stephens, P.J.(1990) Proc Natl Acad Sci U S A 87: 598-602
- PubMed: 2153958
- DOI: https://doi.org/10.1073/pnas.87.2.598
- Primary Citation of Related Structures:
1FD2 - PubMed Abstract:
Azotobacter vinelandii ferredoxin I is a small protein that contains one [4Fe-4S] cluster and one [3Fe-4S] cluster. Recently the x-ray crystal structure has been redetermined and the fdxA gene, which encodes the protein, has been cloned and sequenced. Here we report the site-directed mutation of Cys-20, which is a ligand of the [4Fe-4S] cluster in the native protein, to alanine and the characterization of the protein product by x-ray crystallographic and spectroscopic methods. The data show that the mutant protein again contains one [4Fe-4S] cluster and one [3Fe-4S] cluster. The new [4Fe-4S] cluster obtains its fourth ligand from Cys-24, a free cysteine in the native structure. The formation of this [4Fe-4S] cluster drives rearrangement of the protein structure.
Organizational Affiliation:
Department of Molecular Biology and Biochemistry, University of California, Irvine 92717.