RNase H active site inhibitors of human immunodeficiency virus type 1 reverse transcriptase: design, biochemical activity, and structural information.
Kirschberg, T.A., Balakrishnan, M., Squires, N.H., Barnes, T., Brendza, K.M., Chen, X., Eisenberg, E.J., Jin, W., Kutty, N., Leavitt, S., Liclican, A., Liu, Q., Liu, X., Mak, J., Perry, J.K., Wang, M., Watkins, W.J., Lansdon, E.B.(2009) J Med Chem 52: 5781-5784
- PubMed: 19791799
- DOI: https://doi.org/10.1021/jm900597q
- Primary Citation of Related Structures:
3HYF - PubMed Abstract:
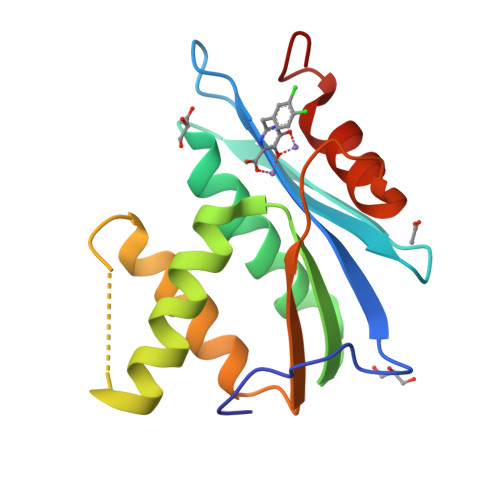
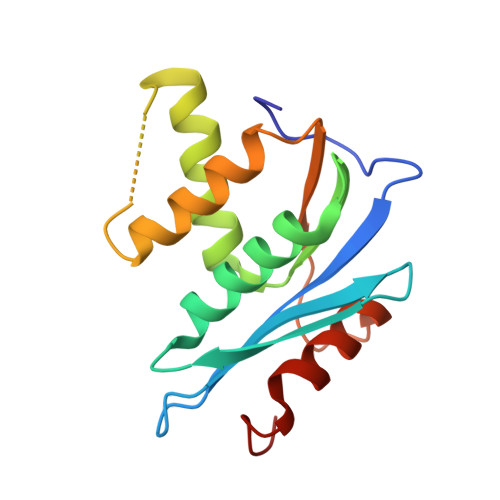
Pyrimidinol carboxylic acids were designed as inhibitors of HIV-1 RNase H function. These molecules can coordinate to two divalent metal ions in the RNase H active site. Inhibition of enzymatic activity was measured in a biochemical assay, but no antiviral effect was observed. Binding was demonstrated via a solid state structure of the isolated p15-Ec domain of HIV-1 RT showing inhibitor and two Mn(II) ions bound to the RNase H active site.
Organizational Affiliation:
Department of Medicinal Chemistry, Gilead Sciences, Foster City, California 94404, USA. tkirschberg@gilead.com