Structural basis for the electron transfer from an open form of NADPH-cytochrome P450 oxidoreductase to heme oxygenase.
Sugishima, M., Sato, H., Higashimoto, Y., Harada, J., Wada, K., Fukuyama, K., Noguchi, M.(2014) Proc Natl Acad Sci U S A 111: 2524-2529
- PubMed: 24550278
- DOI: https://doi.org/10.1073/pnas.1322034111
- Primary Citation of Related Structures:
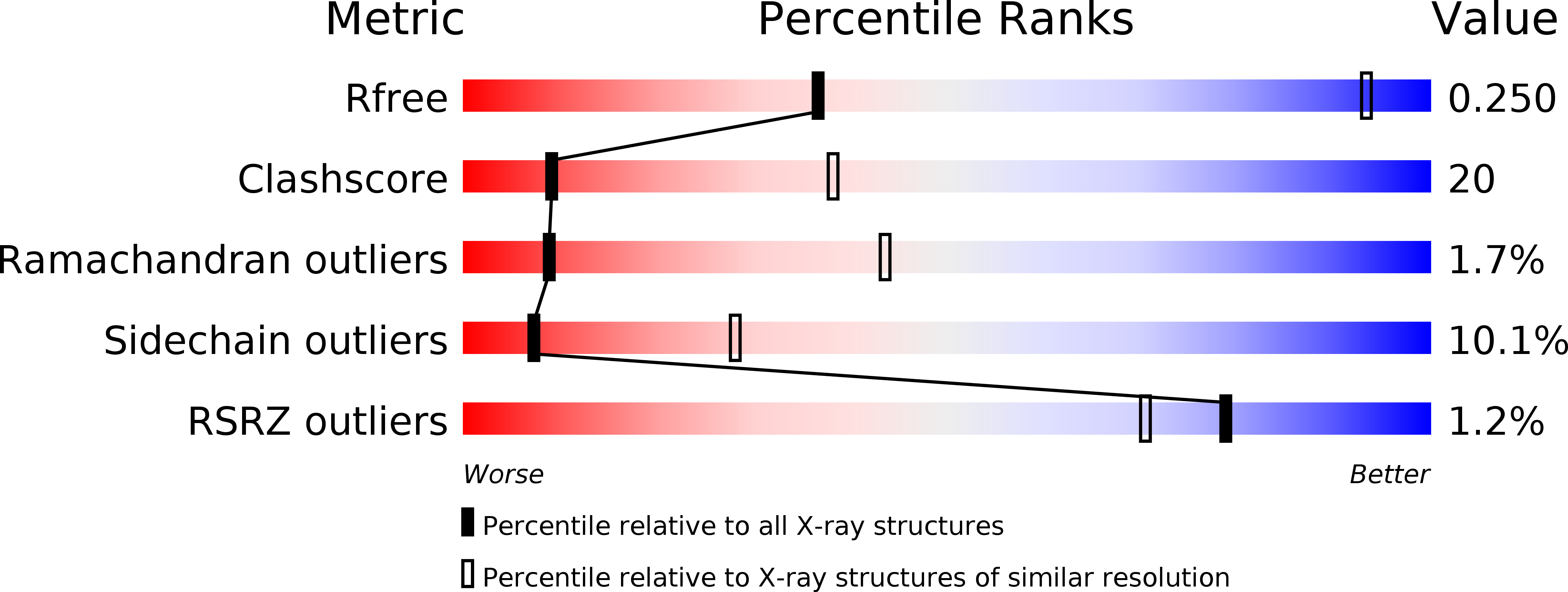
3WKT - PubMed Abstract:
NADPH-cytochrome P450 oxidoreductase (CPR) supplies electrons to various heme proteins including heme oxygenase (HO), which is a key enzyme for heme degradation. Electrons from NADPH flow first to flavin adenine dinucleotide, then to flavin mononucleotide (FMN), and finally to heme in the redox partner. For electron transfer from CPR to its redox partner, the ''closed-open transition'' of CPR is indispensable. Here, we demonstrate that a hinge-shortened CPR variant, which favors an open conformation, makes a stable complex with heme-HO-1 and can support the HO reaction, although its efficiency is extremely limited. Furthermore, we determined the crystal structure of the CPR variant in complex with heme-HO-1 at 4.3-Å resolution. The crystal structure of a complex of CPR and its redox partner was previously unidentified. The distance between heme and FMN in this complex (6 Å) implies direct electron transfer from FMN to heme.
Organizational Affiliation:
Department of Medical Biochemistry and Department of Chemistry, Kurume University School of Medicine, Kurume, Fukuoka 830-0011, Japan.