Crystal structure of ACPA E4 in complex with CII-C-48-CIT
Ge, C., Rikard, H.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Find similar proteins by: Sequence | 3D Structure
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| CII-C-48-CIT | A [auth B] | 18 | Homo sapiens | Mutation(s): 0 |  |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| ACPA E4 Fab fragment - heavy chain | B [auth HHH] | 221 | Homo sapiens | Mutation(s): 0 | 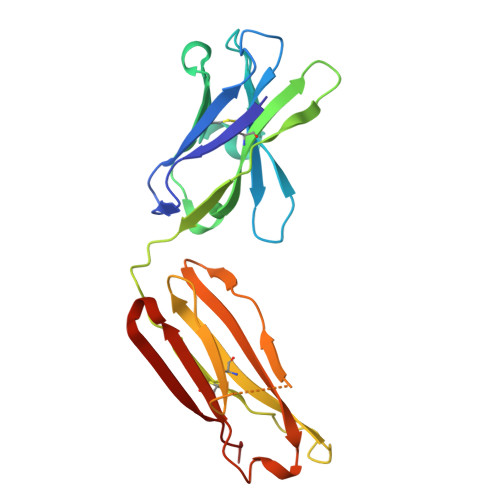 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| ACPA E4 Fab fragment - light chain | C [auth LLL] | 216 | Homo sapiens | Mutation(s): 0 |  |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Glycosylation | |||||
| Glycosylation Sites: 1 | |||||
Sequence AnnotationsExpand | |||||
| |||||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| CIR Query on CIR | A [auth B] | L-PEPTIDE LINKING | C6 H13 N3 O3 |  | ARG |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 77.183 | α = 90 |
| b = 151.774 | β = 90 |
| c = 97.066 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| REFMAC | refinement |
| Aimless | data scaling |
| XDS | data reduction |
| XDS | data scaling |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Swedish Research Council | Sweden | -- |
| Swedish Research Council | Sweden | -- |