Structure-based design of ligands of the m6A-RNA reader YTHDC1
Li, Y., Bedi, R.K., Nai, F., von Roten, V., Dolbois, A., Zalesak, F., Nachawati, R., Huang, D., Caflisch, A.(2022) Eur J Med Chem Rep 5: 100057
Experimental Data Snapshot
Starting Model: experimental
View more details
(2022) Eur J Med Chem Rep 5: 100057
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| YTH domain-containing protein 1 | 183 | Homo sapiens | Mutation(s): 0 Gene Names: YTHDC1, KIAA1966, YT521 | 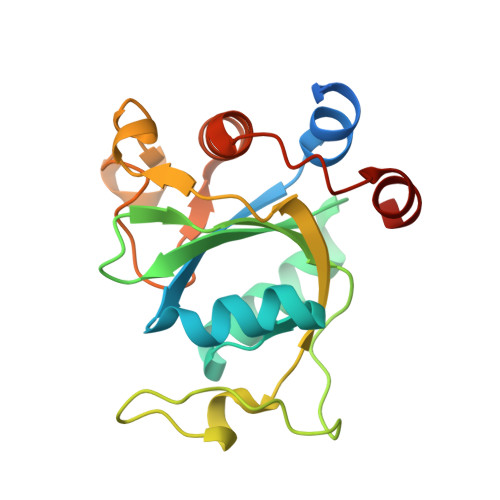 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q96MU7 (Homo sapiens) Explore Q96MU7 Go to UniProtKB: Q96MU7 | |||||
PHAROS: Q96MU7 GTEx: ENSG00000083896 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q96MU7 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| OYN (Subject of Investigation/LOI) Query on OYN | C [auth A], E [auth B] | 2-chloranyl-~{N}-methyl-9~{H}-purin-6-amine C6 H6 Cl N5 RIAVUEFUPHOGJY-UHFFFAOYSA-N |  | ||
| SO4 Query on SO4 | D [auth A], F [auth B], G [auth B], H [auth B], I [auth B] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 39.81 | α = 90 |
| b = 103.6 | β = 105.66 |
| c = 42.03 | γ = 90 |
| Software Name | Purpose |
|---|---|
| XDS | data reduction |
| XSCALE | data scaling |
| PHASER | phasing |
| PHENIX | refinement |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Swiss National Science Foundation | Switzerland | 310030B_189363 |