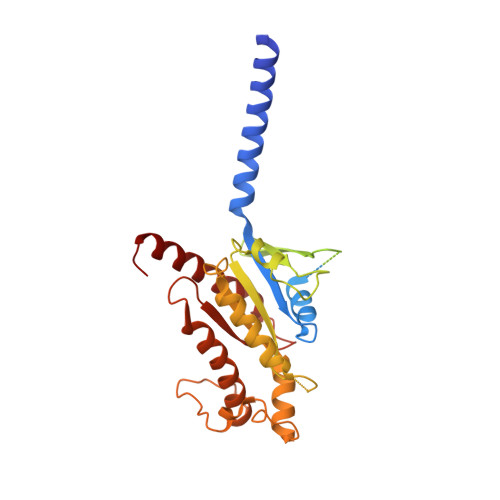
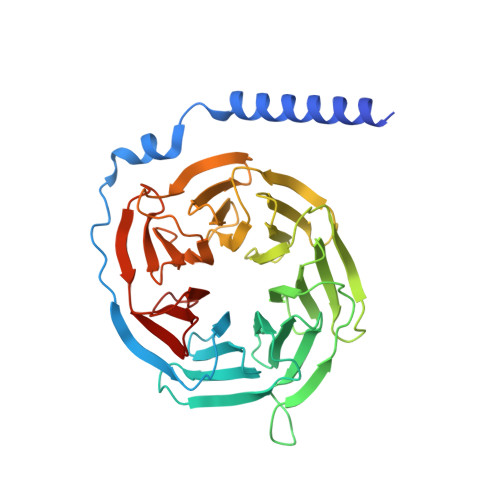
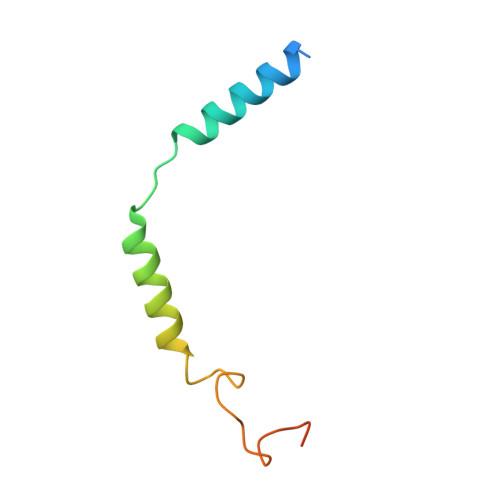
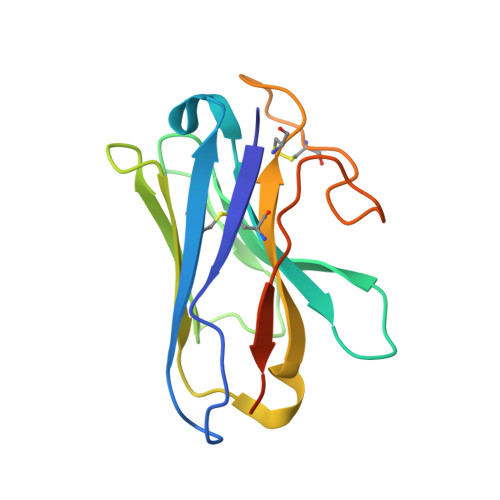
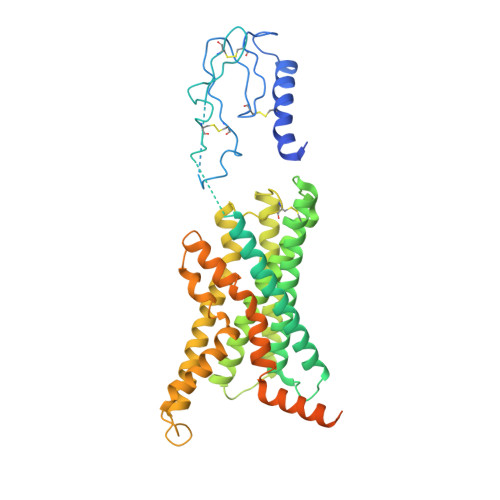
Understanding VPAC receptor family peptide binding and selectivity
Piper, S.J., Deganutti, G., Lu, J., Zhao, P., Liang, Y.L., Lu, Y., Fletcher, M.M., Hossain, M.A., Christopoulos, A., Reynolds, C.A., Danev, R., Sexton, P.M., Wootten, D.(2022) Nat Commun 13: 7013