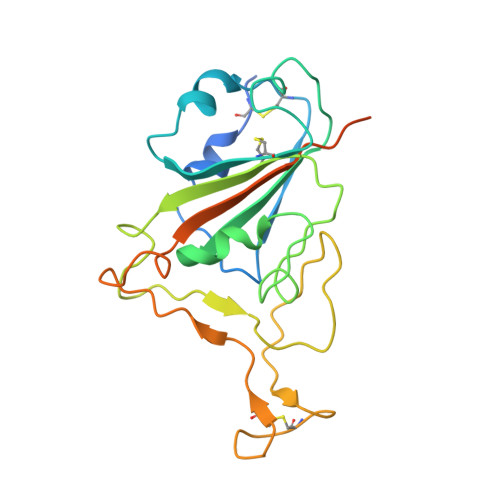
Structural characteristics of BtKY72 RBD bound to bat ACE2 reveal multiple key residues affecting ACE2 usage of sarbecoviruses.
Su, C., He, J., Wang, L., Hu, Y., Cao, J., Bai, B., Qi, J., Gao, G.F., Yang, M., Wang, Q.(2024) mBio 15: e0140424-e0140424
- PubMed: 39082798
- DOI: https://doi.org/10.1128/mbio.01404-24
- Primary Citation of Related Structures:
8K4U - PubMed Abstract:
Two different sarbecoviruses, severe acute respiratory syndrome coronavirus (SARS-CoV) and SARS-CoV-2, have caused serious challenges to public health. Certain sarbecoviruses utilize angiotensin-converting enzyme 2 (ACE2) as their cellular receptor, whereas some do not, speculatively due to the two deletions in their receptor-binding domain (RBD). However, it remains unclear whether sarbecoviruses with one deletion in the RBD can still bind to ACE2. Here, we showed that two phylogenetically related sarbecoviruses with one deletion, BtKY72 and BM48-31, displayed a different ACE2-usage range. The cryo-electron microscopy structure of BtKY72 RBD bound to bat ACE2 identified a key residue important for the interaction between RBD and ACE2. In addition, we demonstrated that the mutations involving four types of core residues enabled the sarbecoviruses with deletion(s) to bind to human ACE2 (hACE2) and broadened the ACE2 usage of SARS-CoV-2. Our findings help predict the potential hACE2-binding ability to emerge sarbecoviruses and develop pan-sarbecovirus therapeutic agents. Many sarbecoviruses, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), possess the ability to bind to receptor angiotensin-converting enzyme 2 (ACE2) through their receptor-binding domain (RBD). However, certain sarbecoviruses with deletion(s) in the RBD lack this capability. In this study, we investigated two closely related short-deletion sarbecoviruses, BtKY72 and BM48-31, and revealed that BtKY72 exhibited a broader ACE2-binding spectrum compared to BM48-31. Structural analysis of the BtKY72 RBD-bat ACE2 complex identifies a critical residue at position 493 contributing to these differences. Furthermore, we demonstrated that the mutations involving four core residues in the RBD enabled the sarbecoviruses with deletion(s) to bind to human ACE2 and expanded the ACE2 usage spectra of SARS-CoV-2. These findings offer crucial insights for accurately predicting the potential threat of newly emerging sarbecoviruses to human health.
Organizational Affiliation:
CAS Key Laboratory of Pathogen Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences (CAS), Beijing, China.