Structure of a human S-adenosylmethionine decarboxylase self-processing ester intermediate and mechanism of putrescine stimulation of processing as revealed by the H243A mutant.
Ekstrom, J.L., Tolbert, W.D., Xiong, H., Pegg, A.E., Ealick, S.E.(2001) Biochemistry 40: 9495-9504
- PubMed: 11583148
- DOI: https://doi.org/10.1021/bi010736o
- Primary Citation of Related Structures:
1JL0 - PubMed Abstract:
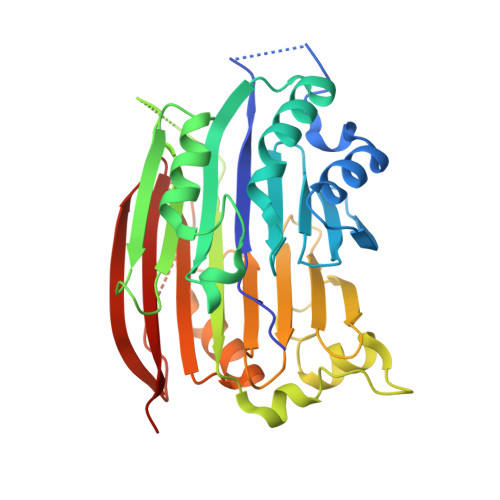
S-Adenosylmethionine decarboxylase (AdoMetDC) is synthesized as a proenzyme that cleaves itself in a putrescine-stimulated reaction via an N-->O acyl shift and beta-elimination to produce an active enzyme with a catalytically essential pyruvoyl residue at the new N-terminus. N-->O acyl shifts initiate the self-processing of other proteins such as inteins and amidohydrolases, but their mechanisms in such proteins are not well understood. We have solved the crystal structure of the H243A mutant of AdoMetDC to 1.5 A resolution. The mutant protein is trapped in the ester form, providing clear evidence for the structure of the ester intermediate in the processing of pyruvoyl enzymes. In addition, a putrescine molecule is bound in a charged region within the beta-sandwich, and cross-links the two beta-sheets through hydrogen bonds to several acidic residues and ordered water molecules. The high-resolution structure provides insight into the mechanism for the self-processing reaction and provides evidence for the mechanism for simulation of the self-processing reaction by putrescine. Studies of the effects of putrescine or 4-aminobutanol on the processing of mutant AdoMetDC proenzymes are consistent with a model in which a single activator molecule interacts with buried Asp174, Glu178, and Glu256, leading to an alteration in the position of Glu11, resulting in stimulation of self-processing.
Organizational Affiliation:
Department of Chemistry and Chemical Biology, Cornell University, Ithaca, New York 14853-1301, USA.