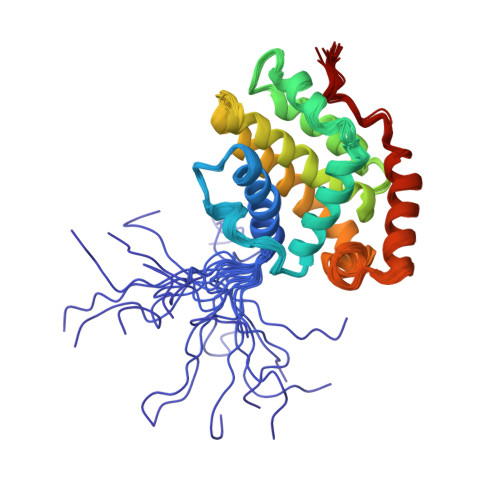
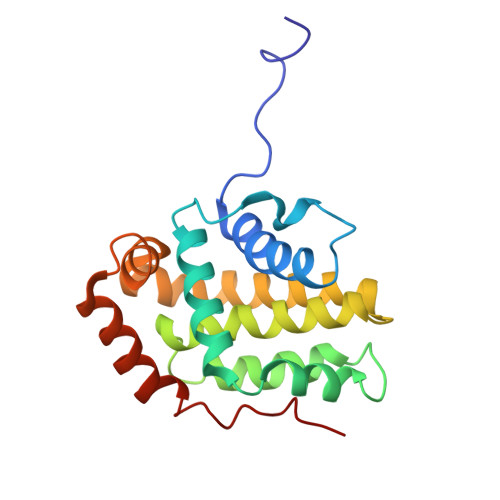
The structure of Bcl-w reveals a role for the C-terminal residues in modulating biological activity
Hinds, M.G., Lackmann, M., Skea, G.L., Harrison, P.J., Huang, D.C.S., Day, C.L.(2003) EMBO J 22: 1497-1507
- PubMed: 12660157
- DOI: https://doi.org/10.1093/emboj/cdg144
- Primary Citation of Related Structures:
1O0L - PubMed Abstract:
Pro-survival Bcl-2-related proteins, critical regulators of apoptosis, contain a hydrophobic groove targeted for binding by the BH3 domain of the pro-apoptotic BH3-only proteins. The solution structure of the pro-survival protein Bcl-w, presented here, reveals that the binding groove is not freely accessible as predicted by previous structures of pro-survival Bcl-2-like molecules. Unexpectedly, the groove appears to be occluded by the C-terminal residues. Binding and kinetic data suggest that the C-terminal residues of Bcl-w and Bcl-x(L) modulate pro-survival activity by regulating ligand access to the groove. Binding of the BH3-only proteins, critical for cell death initiation, is likely to displace the hydrophobic C-terminal region of Bcl-w and Bcl-x(L). Moreover, Bcl-w does not act only by sequestering the BH3-only proteins. There fore, pro-survival Bcl-2-like molecules probably control the activation of downstream effectors by a mechanism that remains to be elucidated.
Organizational Affiliation:
The Walter and Eliza Hall Institute of Medical Research, Melbourne, Victoria 3050, Australia.