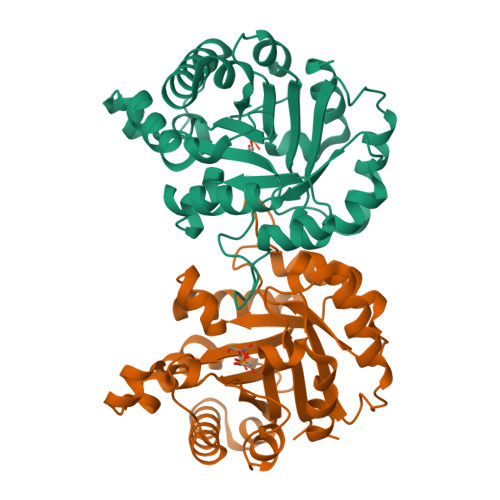
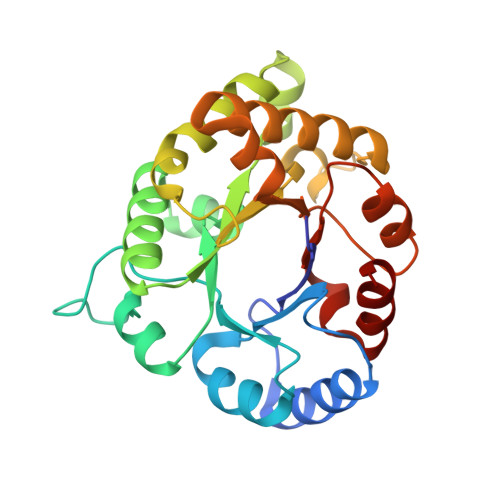
Structure of Plasmodium falciparum Triose-phosphate Isomerase-2-Phosphoglycerate Complex at 1.1-A Resolution
Parthasarathy, S., Eaazhisai, K., Balaram, H., Balaram, P., Murthy, M.R.(2003) J Biological Chem 278: 52461-52470
- PubMed: 14563846
- DOI: https://doi.org/10.1074/jbc.M308525200
- Primary Citation of Related Structures:
1O5X - PubMed Abstract:
Triose-phosphate isomerase, a key enzyme of the glycolytic pathway, catalyzes the isomerization of dihydroxy acetone phosphate and glyceraldehyde 3-phosphate. In this communication we report the crystal structure of Plasmodium falciparum triose-phosphate isomerase complexed to the inhibitor 2-phosphoglycerate at 1.1-A resolution. The crystallographic asymmetric unit contains a dimeric molecule. The inhibitor bound to one of the subunits in which the flexible catalytic loop 6 is in the open conformation has been cleaved into two fragments presumably due to radiation damage. The cleavage products have been tentatively identified as 2-oxoglycerate and meta-phosphate. The intact 2-phosphoglycerate bound to the active site of the other subunit has been observed in two different orientations. The active site loop in this subunit is in both open and "closed" conformations, although the open form is predominant. Concomitant with the loop closure, Phe-96, Leu-167, and residues 208-211 (YGGS) are also observed in dual conformations in the B-subunit. Detailed comparison of the active-site geometry in the present case to the Saccharomyces cerevisiae triose-phosphate isomerase-dihydroxy acetone phosphate and Leishmania mexicana triose-phosphate isomerase-phosphoglycolate complexes, which have also been determined at atomic resolution, shows that certain interactions are common to the three structures, although 2-phosphoglycerate is neither a substrate nor a transition state analogue.
Organizational Affiliation:
Molecular Biophysics Unit, Indian Institute of Science, Bangalore 560 012, India.