Structural and biophysical studies of PCSK9 and its mutants linked to familial hypercholesterolemia.
Cunningham, D., Danley, D.E., Geoghegan, K.F., Griffor, M.C., Hawkins, J.L., Subashi, T.A., Varghese, A.H., Ammirati, M.J., Culp, J.S., Hoth, L.R., Mansour, M.N., McGrath, K.M., Seddon, A.P., Shenolikar, S., Stutzman-Engwall, K.J., Warren, L.C., Xia, D., Qiu, X.(2007) Nat Struct Mol Biol 14: 413-419
- PubMed: 17435765
- DOI: https://doi.org/10.1038/nsmb1235
- Primary Citation of Related Structures:
2P4E - PubMed Abstract:
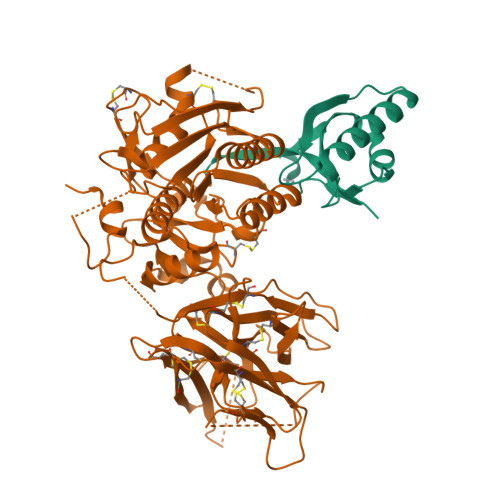
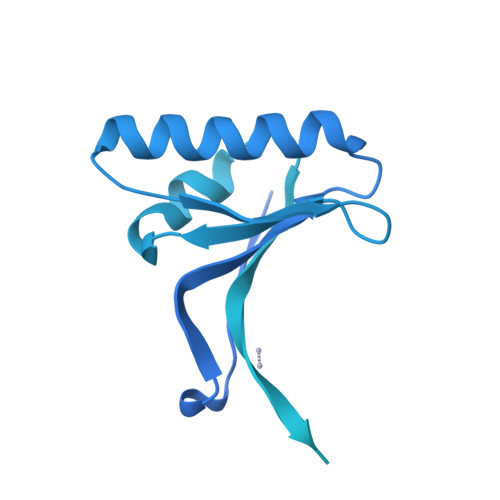
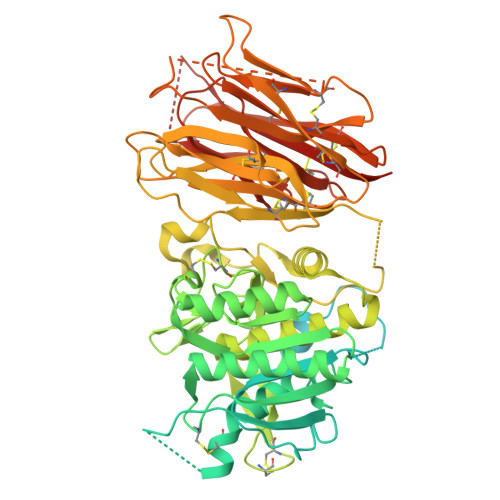
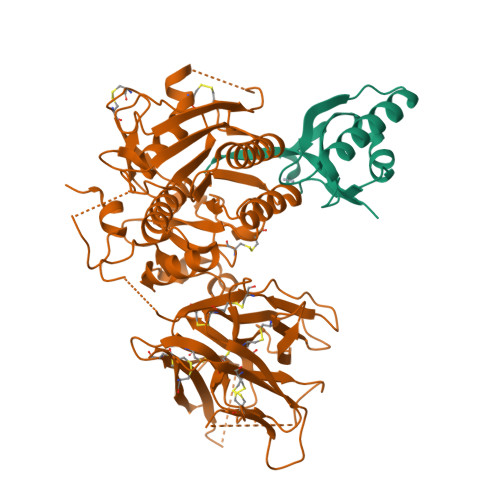
Proprotein convertase subtilisin kexin type 9 (PCSK9) lowers the abundance of surface low-density lipoprotein (LDL) receptor through an undefined mechanism. The structure of human PCSK9 shows the subtilisin-like catalytic site blocked by the prodomain in a noncovalent complex and inaccessible to exogenous ligands, and that the C-terminal domain has a novel fold. Biosensor studies show that PCSK9 binds the extracellular domain of LDL receptor with K(d) = 170 nM at the neutral pH of plasma, but with a K(d) as low as 1 nM at the acidic pH of endosomes. The D374Y gain-of-function mutant, associated with hypercholesterolemia and early-onset cardiovascular disease, binds the receptor 25 times more tightly than wild-type PCSK9 at neutral pH and remains exclusively in a high-affinity complex at the acidic pH. PCSK9 may diminish LDL receptors by a mechanism that requires direct binding but not necessarily receptor proteolysis.
Organizational Affiliation:
Pfizer Inc., Eastern Point Road, Groton, Connecticut 06430, USA.