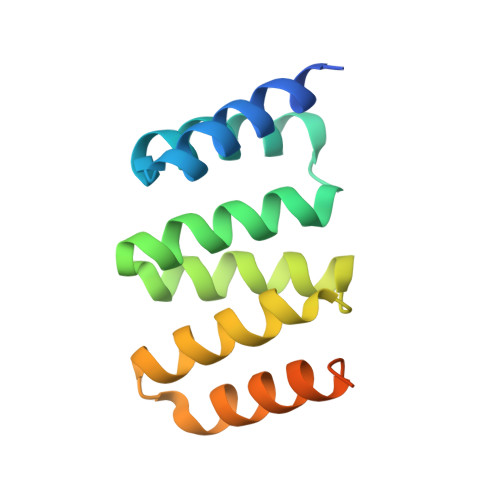
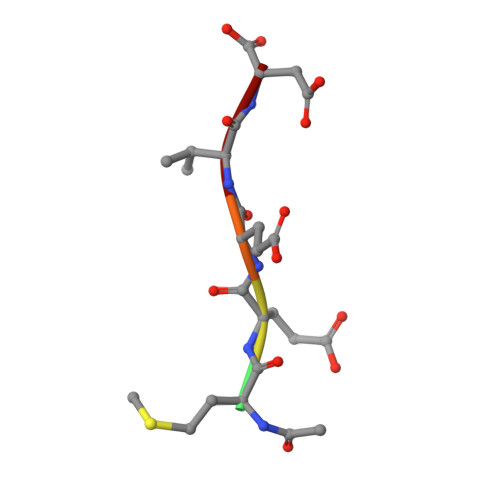
Crystal structure of a designed tetratricopeptide repeat module in complex with its peptide ligand.
Cortajarena, A.L., Wang, J., Regan, L.(2010) FEBS J 277: 1058-1066
- PubMed: 20089039
- DOI: https://doi.org/10.1111/j.1742-4658.2009.07549.x
- Primary Citation of Related Structures:
3KD7 - PubMed Abstract:
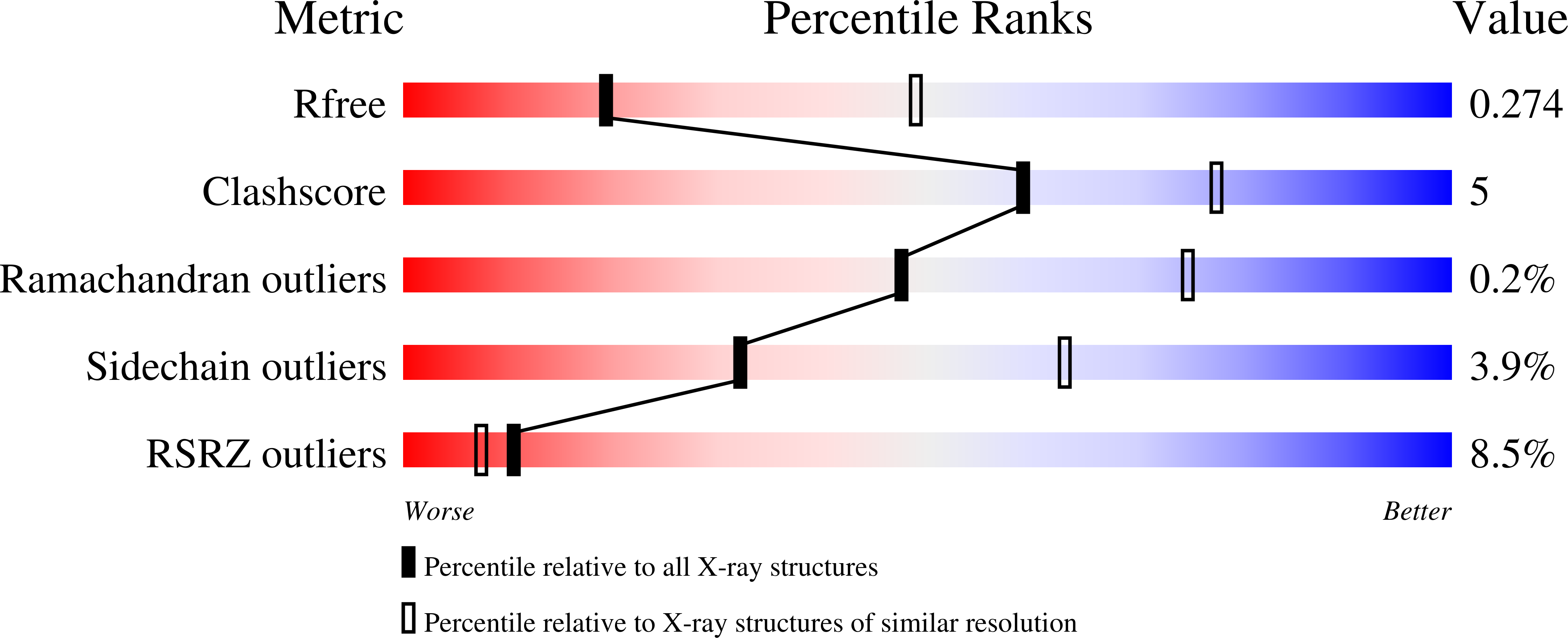
Tetratricopeptide repeats (TPRs) are protein domains that mediate key protein-protein interactions in cells. Several TPR domains bind the C-termini of the chaperones heat shock protein (Hsp)90 and/or Hsp70, and exchange of such binding partners is key for the heat shock response. We have previously described the design of a TPR protein that binds tightly and specifically to the C-terminus of Hsp90, and in doing so, is able to inhibit chaperone function in vivo. Here we present the X-ray crystal structure of the designed TPR domain (CTPR390) in complex with its peptide ligand--the C-terminal residues of Hsp90 (peptide MEEVD). This structure reveals two interesting aspects of the TPR modules. First, a new packing arrangement of 3-TPR modules is observed. The TPR units stack against each other in an unusual fashion to form infinite superhelices in the crystal. Second, the structure provides insights into the molecular basis of TPR-ligand recognition.
Organizational Affiliation:
Department of Molecular Biophysics & Biochemistry, Yale University, New Haven, CT, USA.