Structural definition and substrate specificity of the S28 protease family: the crystal structure of human prolylcarboxypeptidase.
Soisson, S.M., Patel, S.B., Abeywickrema, P.D., Bryne, N.J., Diehl, R.E., Hall, D.L., Ford, R.E., Reid, J.C., Rickert, K.W., Shipman, J.M., Sharma, S., Lumb, K.J.(2010) BMC Struct Biol 10: 16-16
- PubMed: 20540760
- DOI: https://doi.org/10.1186/1472-6807-10-16
- Primary Citation of Related Structures:
3N2Z - PubMed Abstract:
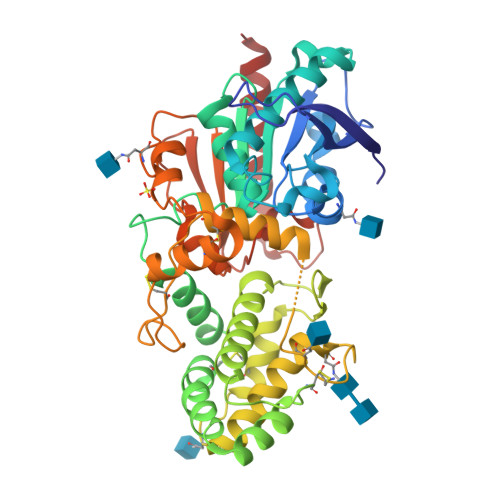
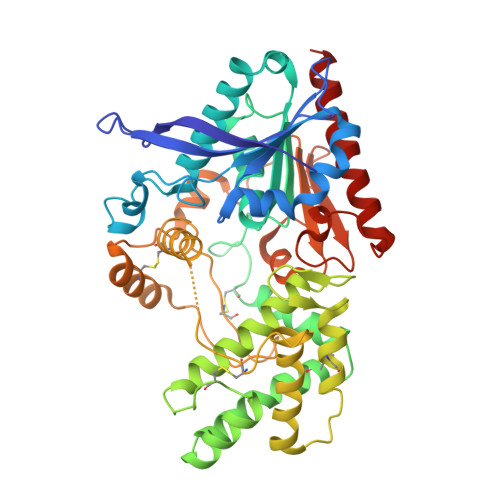
The unique S28 family of proteases is comprised of the carboxypeptidase PRCP and the aminopeptidase DPP7. The structural basis of the different substrate specificities of the two enzymes is not understood nor has the structure of the S28 fold been described. The experimentally phased 2.8 A crystal structure is presented for human PRCP. PRCP contains an alpha/beta hydrolase domain harboring the catalytic Asp-His-Ser triad and a novel helical structural domain that caps the active site. Structural comparisons with prolylendopeptidase and DPP4 identify the S1 proline binding site of PRCP. A structure-based alignment with the previously undescribed structure of DPP7 illuminates the mechanism of orthogonal substrate specificity of PRCP and DPP7. PRCP has an extended active-site cleft that can accommodate proline substrates with multiple N-terminal residues. In contrast, the substrate binding groove of DPP7 is occluded by a short amino-acid insertion unique to DPP7 that creates a truncated active site selective for dipeptidyl proteolysis of N-terminal substrates. The results define the structure of the S28 family of proteases, provide the structural basis of PRCP and DPP7 substrate specificity and enable the rational design of selective PRCP modulators.
Organizational Affiliation:
Global Structural Biology, Merck Research Laboratories, P,O, Box 4, West Point, PA 19486, USA. stephen_soisson@merck.com