Integrated description of protein dynamics from room-temperature X-ray crystallography and NMR.
Fenwick, R.B., van den Bedem, H., Fraser, J.S., Wright, P.E.(2014) Proc Natl Acad Sci U S A 111: E445-E454
- PubMed: 24474795
- DOI: https://doi.org/10.1073/pnas.1323440111
- Primary Citation of Related Structures:
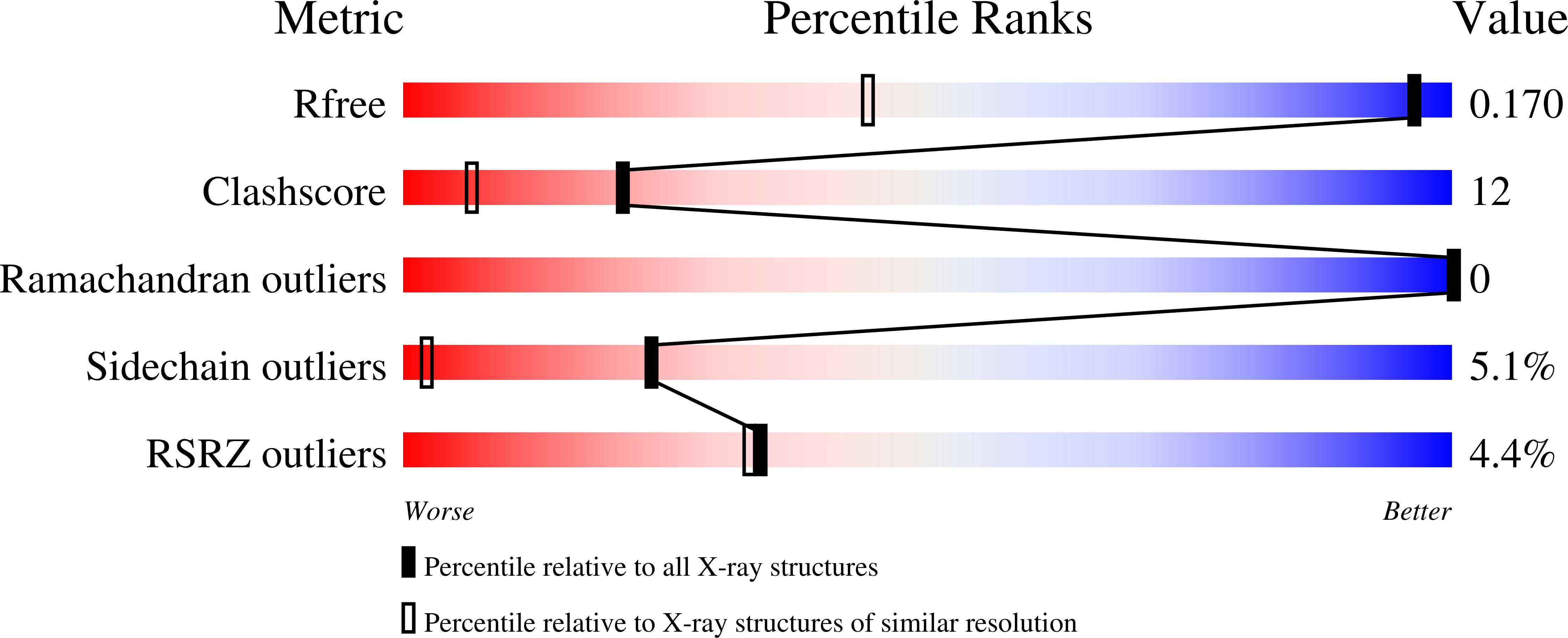
4NX6, 4NX7 - PubMed Abstract:
Detailed descriptions of atomic coordinates and motions are required for an understanding of protein dynamics and their relation to molecular recognition, catalytic function, and allostery. Historically, NMR relaxation measurements have played a dominant role in the determination of the amplitudes and timescales (picosecond-nanosecond) of bond vector fluctuations, whereas high-resolution X-ray diffraction experiments can reveal the presence of and provide atomic coordinates for multiple, weakly populated substates in the protein conformational ensemble. Here we report a hybrid NMR and X-ray crystallography analysis that provides a more complete dynamic picture and a more quantitative description of the timescale and amplitude of fluctuations in atomic coordinates than is obtainable from the individual methods alone. Order parameters (S(2)) were calculated from single-conformer and multiconformer models fitted to room temperature and cryogenic X-ray diffraction data for dihydrofolate reductase. Backbone and side-chain order parameters derived from NMR relaxation experiments are in excellent agreement with those calculated from the room-temperature single-conformer and multiconformer models, showing that the picosecond timescale motions observed in solution occur also in the crystalline state. These motions are quenched in the crystal at cryogenic temperatures. The combination of NMR and X-ray crystallography in iterative refinement promises to provide an atomic resolution description of the alternate conformational substates that are sampled through picosecond to nanosecond timescale fluctuations of the protein structure. The method also provides insights into the structural heterogeneity of nonmethyl side chains, aromatic residues, and ligands, which are less commonly analyzed by NMR relaxation measurements.
Organizational Affiliation:
Department of Integrative Structural and Computational Biology and the Skaggs Institute for Chemical Biology, The Scripps Research Institute, La Jolla, CA 92037.