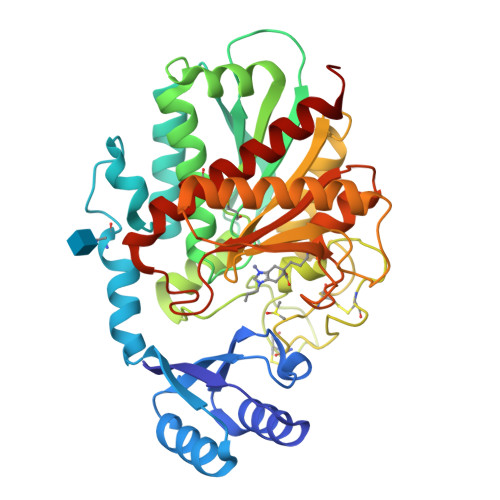
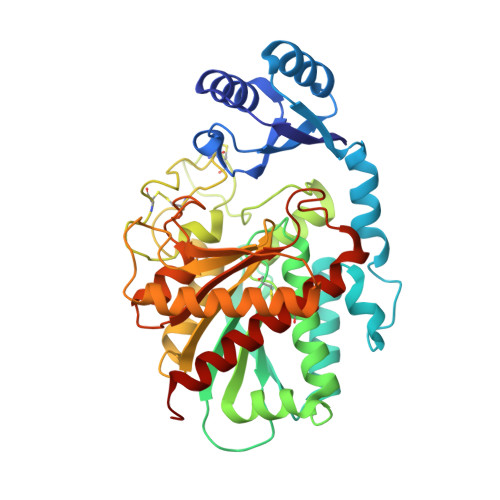
Design and synthesis of conformationally restricted inhibitors of active thrombin activatable fibrinolysis inhibitor (TAFIa).
Brink, M., Dahlen, A., Olsson, T., Polla, M., Svensson, T.(2014) Bioorg Med Chem 22: 2261-2268
- PubMed: 24588961
- DOI: https://doi.org/10.1016/j.bmc.2014.02.010
- Primary Citation of Related Structures:
4P10 - PubMed Abstract:
A series of 4,5,6,7-tetrahydro-1H-benzimidazole-5-carboxylic acid and 5,6,7,8-tetrahydroimidazo[1,2-a]pyridine-7-carboxylic acid derivatives designed as inhibitors of TAFIa has been prepared via a common hydrogenation-alkylation sequence starting from the appropriate benzimidazole and imidazopyridine system. We present a successful design strategy using a conformational restriction approach resulting in potent and selective inhibitors of TAFIa. The X-ray structure of compound 5 in complex with a H333Y/H335Q double mutant TAFI indicate that the conformational restriction is responsible for the observed potency increase.
Organizational Affiliation:
Cardiovascular and Metabolic Disorders Research Area, AstraZeneca R&D Mölndal, SE-431 83 Mölndal, Sweden.