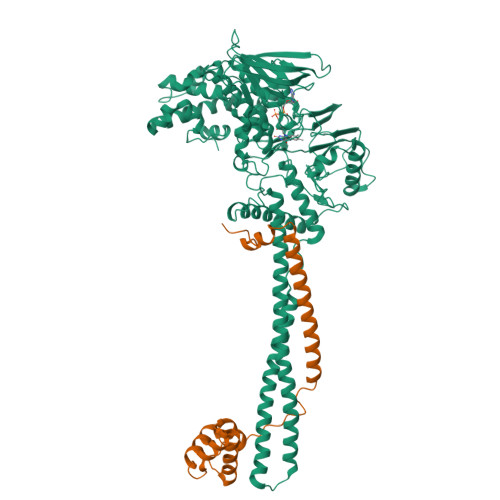
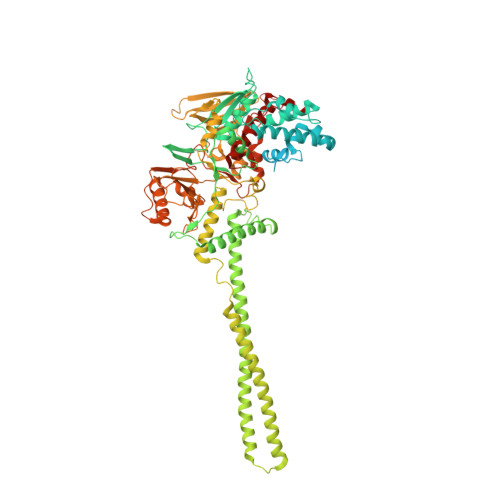
LSD1/KDM1A mutations associated to a newly described form of intellectual disability impair demethylase activity and binding to transcription factors.
Pilotto, S., Speranzini, V., Marabelli, C., Rusconi, F., Toffolo, E., Grillo, B., Battaglioli, E., Mattevi, A.(2016) Hum Mol Genet 25: 2578-2587
- PubMed: 27094131
- DOI: https://doi.org/10.1093/hmg/ddw120
- Primary Citation of Related Structures:
5L3B, 5L3C, 5L3D - PubMed Abstract:
Genetic diseases often lead to rare and severe syndromes and the identification of the genetic and protein alterations responsible for the pathogenesis is essential to understand both the physiological and pathological role of the gene product. Recently, de novo variants have been mapped on the gene encoding for the lysine-specific histone demethylase 1 (LSD1)/lysine(K)-specific histone demethylase 1A in three patients characterized by a new genetic disorder. We have analyzed the effects of these pathological mutations on the structure, stability and activity of LSD1 using both in vitro and cellular approaches. The three mutations (Glu403Lys, Asp580Gly and Tyr785His) affect active-site residues and lead to a partial impairment of catalytic activity. They also differentially perturb the ability of LSD1 to engage transcription factors that orchestrate key developmental programs. Moreover, cellular data indicate a decrease in the protein cellular half-life. Taken together, these results demonstrate the relevance of LSD1 in gene regulation and how even moderate alterations in its stability, catalytic activity and binding properties can strongly affect organism development. This depicts a perturbed interplay of catalytic and non-catalytic processes at the origin of the pathology.
Organizational Affiliation:
Department of Biology and Biotechnology, University of Pavia, 27100 Pavia, Italy.