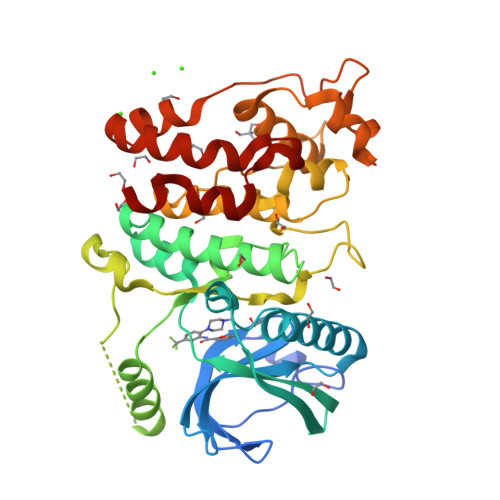
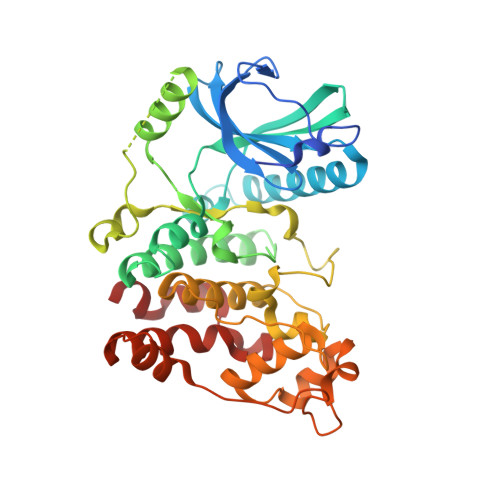
Development of Potent, Selective SRPK1 Inhibitors as Potential Topical Therapeutics for Neovascular Eye Disease.
Batson, J., Toop, H.D., Redondo, C., Babaei-Jadidi, R., Chaikuad, A., Wearmouth, S.F., Gibbons, B., Allen, C., Tallant, C., Zhang, J., Du, C., Hancox, J.C., Hawtrey, T., Da Rocha, J., Griffith, R., Knapp, S., Bates, D.O., Morris, J.C.(2017) ACS Chem Biol 12: 825-832
- PubMed: 28135068
- DOI: https://doi.org/10.1021/acschembio.6b01048
- Primary Citation of Related Structures:
5MXX, 5MY8, 5MYV - PubMed Abstract:
Serine/arginine-protein kinase 1 (SRPK1) regulates alternative splicing of VEGF-A to pro-angiogenic isoforms and SRPK1 inhibition can restore the balance of pro/antiangiogenic isoforms to normal physiological levels. The lack of potency and selectivity of available compounds has limited development of SRPK1 inhibitors, with the control of alternative splicing by splicing factor-specific kinases yet to be translated. We present here compounds that occupy a binding pocket created by the unique helical insert of SRPK1, and trigger a backbone flip in the hinge region, that results in potent (<10 nM) and selective inhibition of SRPK1 kinase activity. Treatment with these inhibitors inhibited SRPK1 activity and phosphorylation of serine/arginine splicing factor 1 (SRSF1), resulting in alternative splicing of VEGF-A from pro-angiogenic to antiangiogenic isoforms. This property resulted in potent inhibition of blood vessel growth in models of choroidal angiogenesis in vivo. This work identifies tool compounds for splice isoform selective targeting of pro-angiogenic VEGF, which may lead to new therapeutic strategies for a diversity of diseases where dysfunctional splicing drives disease development.
Organizational Affiliation:
Exonate Ltd , Unit 23, Cambridge Science Park, Cambridge, United Kingdom.