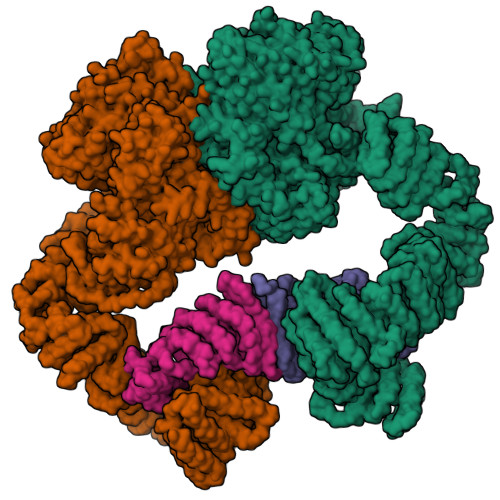
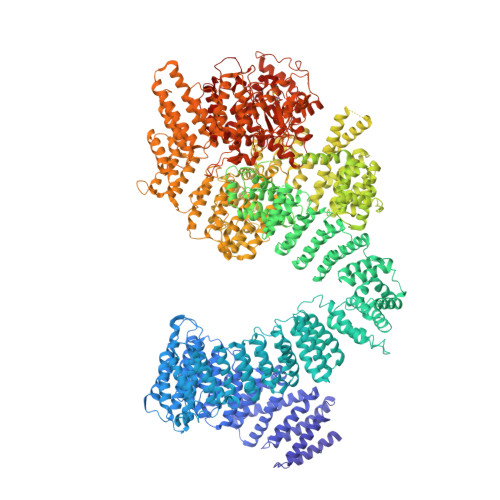
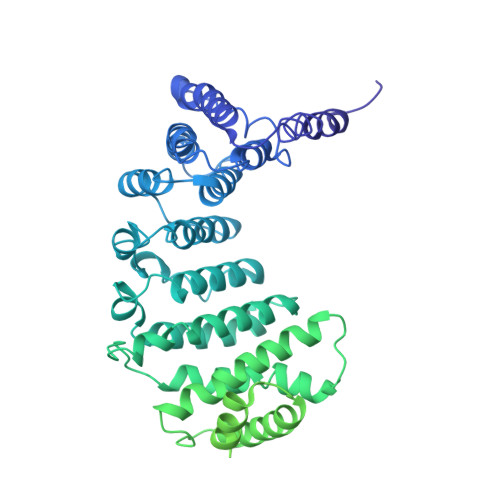
Cryo-EM structure of human ATR-ATRIP complex.
Rao, Q., Liu, M., Tian, Y., Wu, Z., Hao, Y., Song, L., Qin, Z., Ding, C., Wang, H.W., Wang, J., Xu, Y.(2018) Cell Res 28: 143-156
- PubMed: 29271416
- DOI: https://doi.org/10.1038/cr.2017.158
- Primary Citation of Related Structures:
5YZ0 - PubMed Abstract:
ATR (ataxia telangiectasia-mutated and Rad3-related) protein kinase and ATRIP (ATR-interacting protein) form a complex and play a critical role in response to replication stress and DNA damage. Here, we determined the cryo-electron microscopy (EM) structure of the human ATR-ATRIP complex at 4.7 Å resolution and built an atomic model of the C-terminal catalytic core of ATR (residues 1 521-2 644) at 3.9 Å resolution. The complex adopts a hollow "heart" shape, consisting of two ATR monomers in distinct conformations. The EM map for ATRIP reveals 14 HEAT repeats in an extended "S" shape. The conformational flexibility of ATR allows ATRIP to properly lock the N-termini of the two ATR monomers to favor ATR-ATRIP complex formation and functional diversity. The isolated "head-head" and "tail-tail" each adopts a pseudo 2-fold symmetry. The catalytic pockets face outward and substrate access is not restricted by inhibitory elements. Our studies provide a structural basis for understanding the assembly of the ATR-ATRIP complex and a framework for characterizing ATR-mediated DNA repair pathways.
Organizational Affiliation:
Fudan University Shanghai Cancer Center, Institute of Biomedical Sciences, Shanghai Medical College of Fudan University, Shanghai 200032, China.