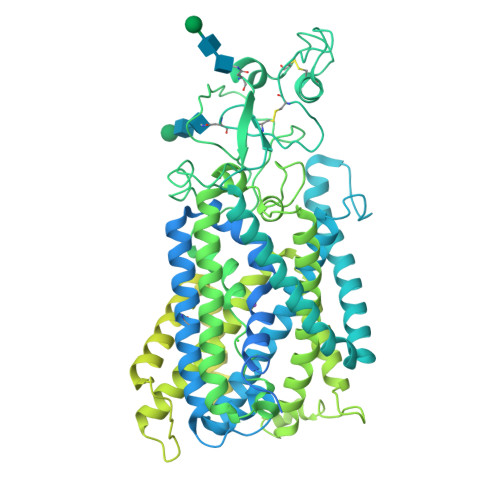
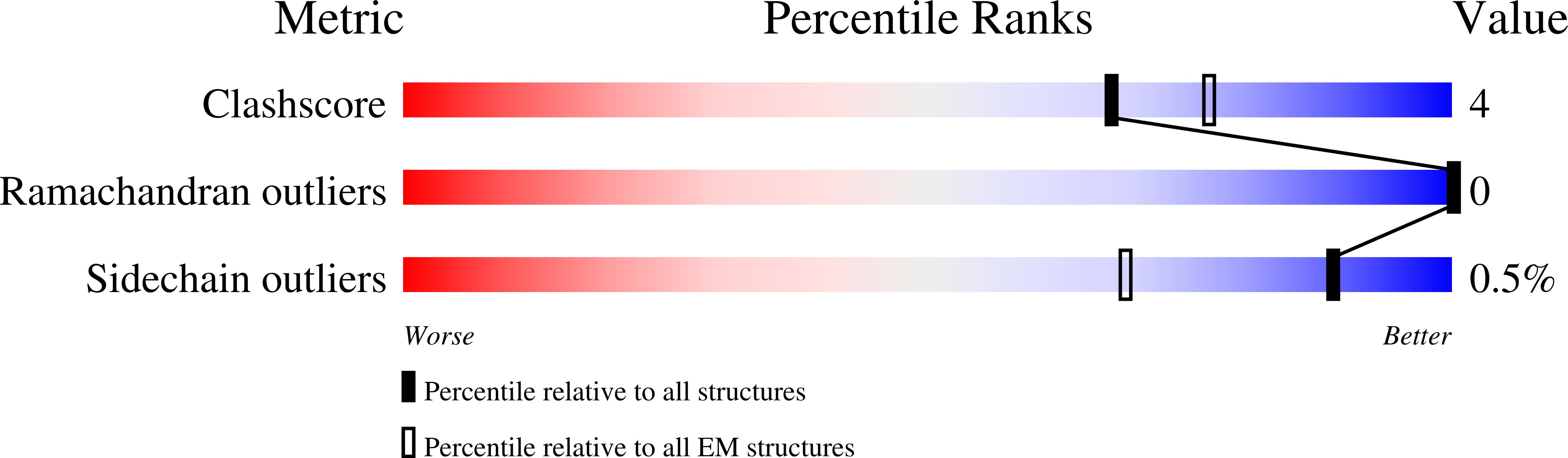Cryo-EM structure of the potassium-chloride cotransporter KCC4 in lipid nanodiscs.
Reid, M.S., Kern, D.M., Brohawn, S.G.(2020) Elife 9
- PubMed: 32286222
- DOI: https://doi.org/10.7554/eLife.52505
- Primary Citation of Related Structures:
6UKN - PubMed Abstract:
Cation-chloride-cotransporters (CCCs) catalyze transport of Cl - with K + and/or Na + across cellular membranes. CCCs play roles in cellular volume regulation, neural development and function, audition, regulation of blood pressure, and renal function. CCCs are targets of clinically important drugs including loop diuretics and their disruption has been implicated in pathophysiology including epilepsy, hearing loss, and the genetic disorders Andermann, Gitelman, and Bartter syndromes. Here we present the structure of a CCC, the Mus musculus K + -Cl - cotransporter (KCC) KCC4, in lipid nanodiscs determined by cryo-EM. The structure, captured in an inside-open conformation, reveals the architecture of KCCs including an extracellular domain poised to regulate transport activity through an outer gate. We identify binding sites for substrate K + and Cl - ions, demonstrate the importance of key coordinating residues for transporter activity, and provide a structural explanation for varied substrate specificity and ion transport ratio among CCCs. These results provide mechanistic insight into the function and regulation of a physiologically important transporter family.
Organizational Affiliation:
Department of Molecular and Cell Biology, University of California Berkeley, Berkeley, United States.