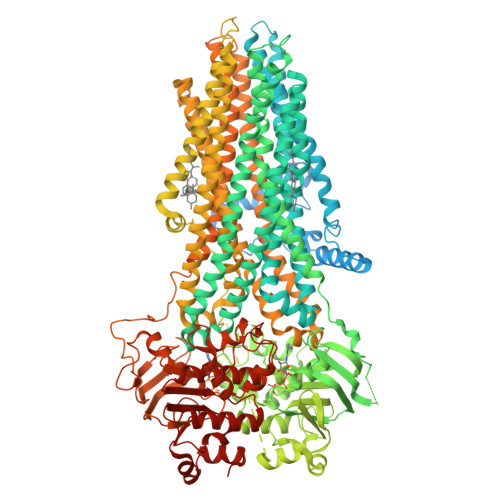
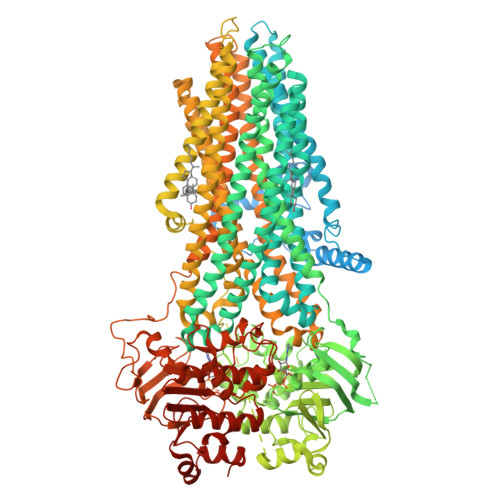
Characterization of the kinetic cycle of an ABC transporter by single-molecule and cryo-EM analyses.
Wang, L., Johnson, Z.L., Wasserman, M.R., Levring, J., Chen, J., Liu, S.(2020) Elife 9
- PubMed: 32458799
- DOI: https://doi.org/10.7554/eLife.56451
- Primary Citation of Related Structures:
6UY0 - PubMed Abstract:
ATP-binding cassette (ABC) transporters are molecular pumps ubiquitous across all kingdoms of life. While their structures have been widely reported, the kinetics governing their transport cycles remain largely unexplored. Multidrug resistance protein 1 (MRP1) is an ABC exporter that extrudes a variety of chemotherapeutic agents and native substrates. Previously, the structures of MRP1 were determined in an inward-facing (IF) or outward-facing (OF) conformation. Here, we used single-molecule fluorescence spectroscopy to track the conformational changes of bovine MRP1 (bMRP1) in real time. We also determined the structure of bMRP1 under active turnover conditions. Our results show that substrate stimulates ATP hydrolysis by accelerating the IF-to-OF transition. The rate-limiting step of the transport cycle is the dissociation of the nucleotide-binding-domain dimer, while ATP hydrolysis per se does not reset MRP1 to the resting state. The combination of structural and kinetic data illustrates how different conformations of MRP1 are temporally linked and how substrate and ATP alter protein dynamics to achieve active transport.
Organizational Affiliation:
Laboratory of Nanoscale Biophysics and Biochemistry, The Rockefeller University, New York, United States.