Coupling of Ca2+and voltage activation in BK channels through the alpha B helix/voltage sensor interface.
Geng, Y., Deng, Z., Zhang, G., Budelli, G., Butler, A., Yuan, P., Cui, J., Salkoff, L., Magleby, K.L.(2020) Proc Natl Acad Sci U S A 117: 14512-14521
- PubMed: 32513714
- DOI: https://doi.org/10.1073/pnas.1908183117
- Primary Citation of Related Structures:
6V5A - PubMed Abstract:
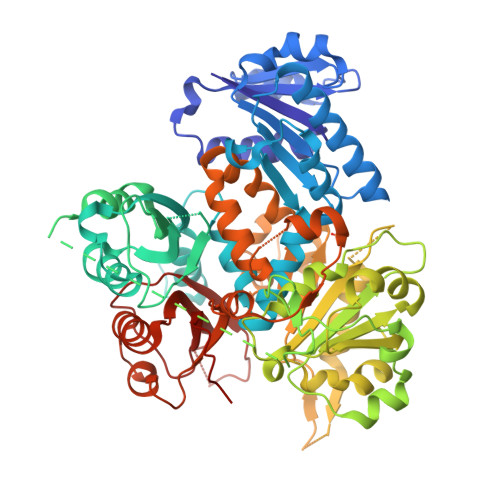
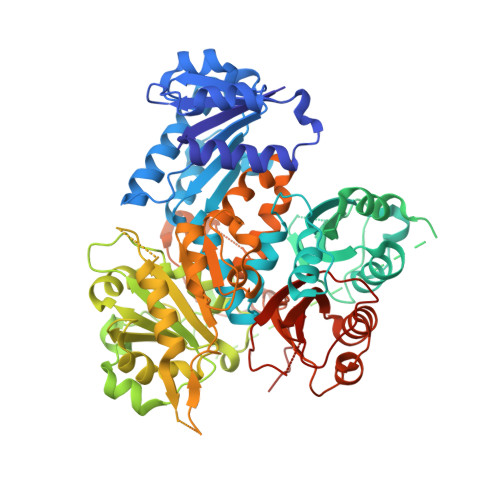
Large-conductance Ca 2+ and voltage-activated K + (BK) channels control membrane excitability in many cell types. BK channels are tetrameric. Each subunit is composed of a voltage sensor domain (VSD), a central pore-gate domain, and a large cytoplasmic domain (CTD) that contains the Ca 2+ sensors. While it is known that BK channels are activated by voltage and Ca 2+ , and that voltage and Ca 2+ activations interact, less is known about the mechanisms involved. We explore here these mechanisms by examining the gating contribution of an interface formed between the VSDs and the αB helices located at the top of the CTDs. Proline mutations in the αB helix greatly decreased voltage activation while having negligible effects on gating currents. Analysis with the Horrigan, Cui, and Aldrich model indicated a decreased coupling between voltage sensors and pore gate. Proline mutations decreased Ca 2+ activation for both Ca 2+ bowl and RCK1 Ca 2+ sites, suggesting that both high-affinity Ca 2+ sites transduce their effect, at least in part, through the αB helix. Mg 2+ activation also decreased. The crystal structure of the CTD with proline mutation L390P showed a flattening of the first helical turn in the αB helix compared to wild type, without other notable differences in the CTD, indicating that structural changes from the mutation were confined to the αB helix. These findings indicate that an intact αB helix/VSD interface is required for effective coupling of Ca 2+ binding and voltage depolarization to pore opening and that shared Ca 2+ and voltage transduction pathways involving the αB helix may be involved.
Organizational Affiliation:
Department of Physiology and Biophysics, University of Miami Miller School of Medicine, Miami FL 33136.