Structure of human FAPalpha in complex with linagliptin
Nar, H., Schnapp, G., Schreiner, P.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Prolyl endopeptidase FAP | 722 | Homo sapiens | Mutation(s): 0 Gene Names: FAP EC: 3.4.21.26 (PDB Primary Data), 3.4.14.5 (PDB Primary Data), 3.4.21 (PDB Primary Data) | 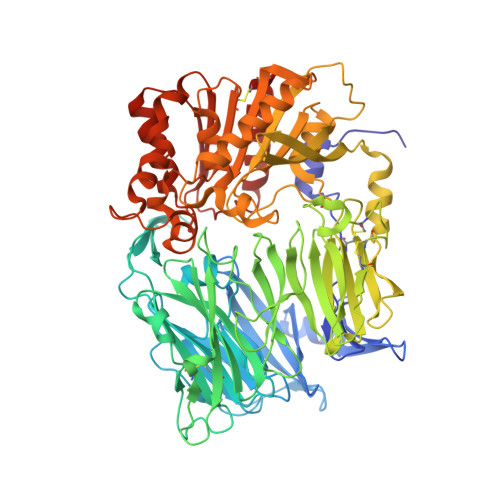 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q12884 (Homo sapiens) Explore Q12884 Go to UniProtKB: Q12884 | |||||
PHAROS: Q12884 GTEx: ENSG00000078098 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q12884 | ||||
Glycosylation | |||||
| Glycosylation Sites: 4 | Go to GlyGen: Q12884-1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | 2D Diagram | Glycosylation | 3D Interactions |
| 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | E, G, H, I, J | 2 |  | N-Glycosylation | |
Glycosylation Resources | |||||
GlyTouCan: G42666HT GlyCosmos: G42666HT GlyGen: G42666HT | |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| 356 (Subject of Investigation/LOI) Query on 356 | Q [auth A], S [auth B], U [auth C], W [auth D] | 8-[(3R)-3-Aminopiperidin-1-yl]-7-but-2-yn-1-yl-3-methyl-1-[(4-methylquinazolin-2-yl)methyl]-3,7-dihydro-1H-purine-2,6-d
ione C25 H28 N8 O2 LTXREWYXXSTFRX-QGZVFWFLSA-N |  | ||
| NAG Query on NAG | N [auth A] O [auth A] P [auth A] R [auth B] T [auth C] | 2-acetamido-2-deoxy-beta-D-glucopyranose C8 H15 N O6 OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 67.586 | α = 90 |
| b = 253.604 | β = 89.76 |
| c = 115.247 | γ = 90 |
| Software Name | Purpose |
|---|---|
| XDS | data reduction |
| BUSTER | refinement |
| PDB_EXTRACT | data extraction |
| XSCALE | data scaling |
| MOLREP | phasing |