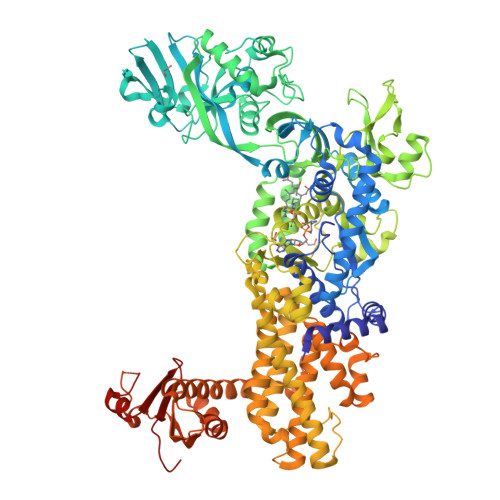
Inhibitory mechanism of reveromycin A at the tRNA binding site of a class I synthetase.
Chen, B., Luo, S., Zhang, S., Ju, Y., Gu, Q., Xu, J., Yang, X.L., Zhou, H.(2021) Nat Commun 12: 1616-1616
- PubMed: 33712620
- DOI: https://doi.org/10.1038/s41467-021-21902-0
- Primary Citation of Related Structures:
7D5C - PubMed Abstract:
The polyketide natural product reveromycin A (RM-A) exhibits antifungal, anticancer, anti-bone metastasis, anti-periodontitis and anti-osteoporosis activities by selectively inhibiting eukaryotic cytoplasmic isoleucyl-tRNA synthetase (IleRS). Herein, a co-crystal structure suggests that the RM-A molecule occupies the substrate tRNA Ile binding site of Saccharomyces cerevisiae IleRS (ScIleRS), by partially mimicking the binding of tRNA Ile . RM-A binding is facilitated by the copurified intermediate product isoleucyl-adenylate (Ile-AMP). The binding assays confirm that RM-A competes with tRNA Ile while binding synergistically with L-isoleucine or intermediate analogue Ile-AMS to the aminoacylation pocket of ScIleRS. This study highlights that the vast tRNA binding site of the Rossmann-fold catalytic domain of class I aminoacyl-tRNA synthetases could be targeted by a small molecule. This finding will inform future rational drug design.
Organizational Affiliation:
Guangdong Provincial Key Laboratory of Chiral Molecule and Drug Discovery, School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou, 510006, China.