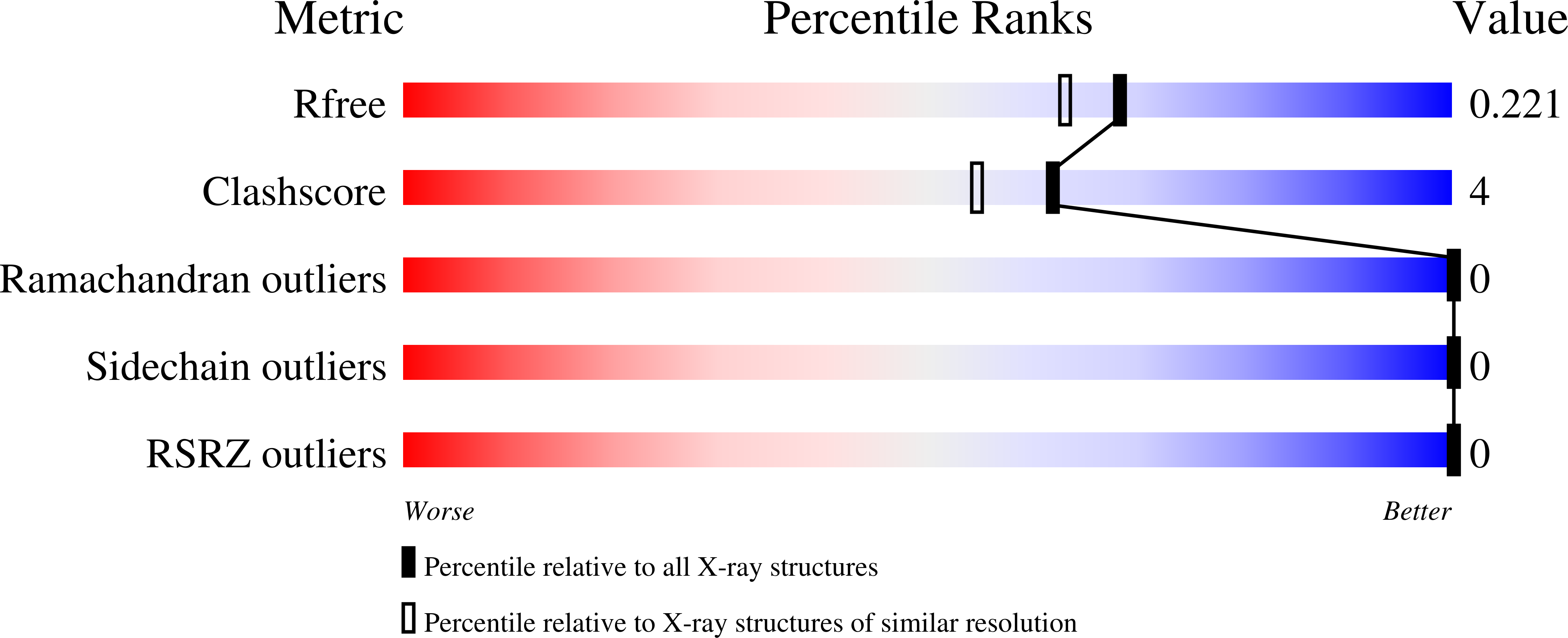
The N-terminal length and side-chain composition of CXCL13 affect crystallization, structure and functional activity.
Rosenberg Jr., E.M., Herrington, J., Rajasekaran, D., Murphy, J.W., Pantouris, G., Lolis, E.J.(2020) Acta Crystallogr D Struct Biol 76: 1033-1049
- PubMed: 33021505
- DOI: https://doi.org/10.1107/S2059798320011687
- Primary Citation of Related Structures:
6VGJ, 7JNY - PubMed Abstract:
CXCL13 is the cognate chemokine agonist of CXCR5, a class A G-protein-coupled receptor (GPCR) that is essential for proper humoral immune responses. Using a `methionine scanning' mutagenesis method on the N-terminus of CXCL13, which is the chemokine signaling region, it was shown that minor length alterations and side-chain substitutions still result in CXCR5 activation. This observation indicates that the orthosteric pocket of CXCR5 can tolerate these changes without severely affecting the activity. The introduction of bulk on the ligand was well tolerated by the receptor, whereas a loss of contacts was less tolerated. Furthermore, two crystal structures of CXCL13 mutants were solved, both of which represent the first uncomplexed structures of the human protein. These structures were stabilized by unique interactions formed by the N-termini of the ligands, indicating that CXCL13 exhibits substantial N-terminal flexibility while the chemokine core domain remains largely unchanged. Additionally, it was observed that CXCL13 harbors a large degree of flexibility in the C-terminal extension of the ligand. Comparisons with other published structures of human and murine CXCL13 validate the relative rigidity of the core domain as well as the N- and C-terminal mobilities. Collectively, these mutants and their structures provide the field with additional insights into how CXCL13 interacts with CXCR5.
Organizational Affiliation:
Department of Pharmacology, Yale School of Medicine, New Haven, CT 06520, USA.