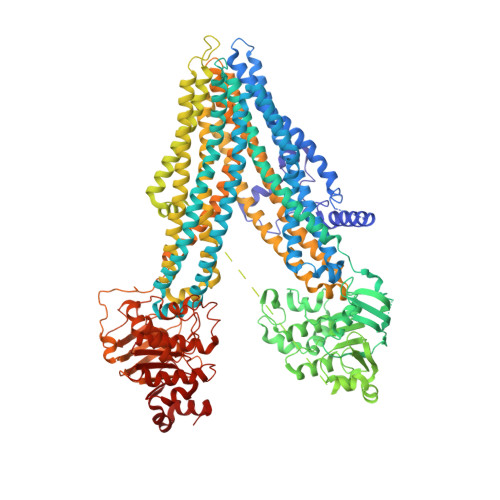
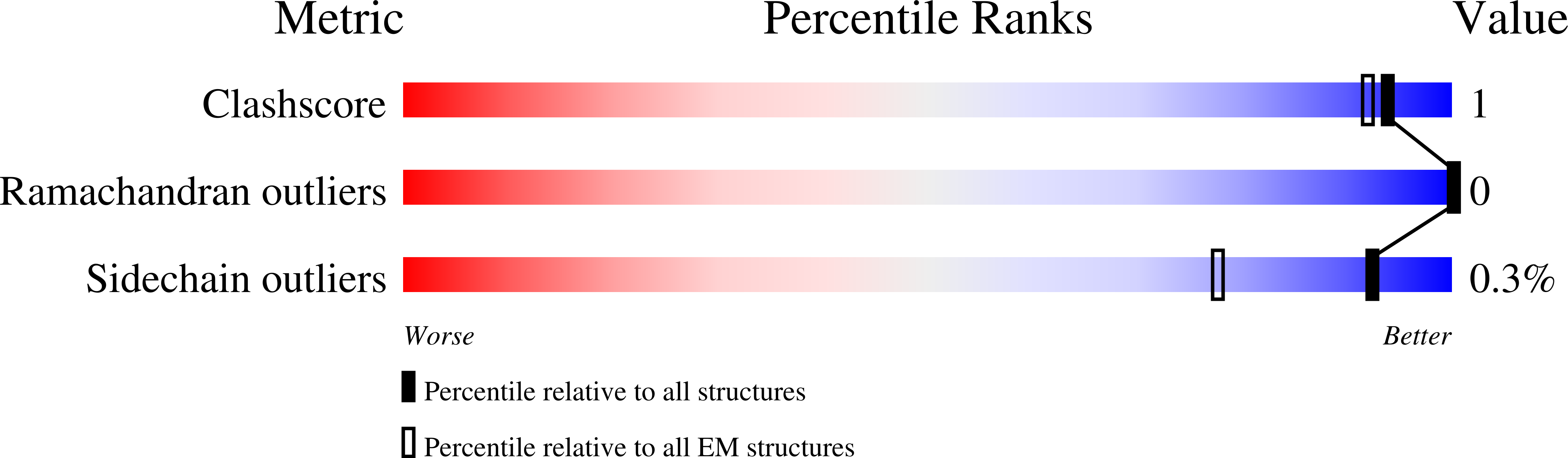A macrocyclic peptide inhibitor traps MRP1 in a catalytically incompetent conformation.
Pietz, H.L., Abbas, A., Johnson, Z.L., Oldham, M.L., Suga, H., Chen, J.(2023) Proc Natl Acad Sci U S A 120: e2220012120-e2220012120
- PubMed: 36893260
- DOI: https://doi.org/10.1073/pnas.2220012120
- Primary Citation of Related Structures:
8F4B - PubMed Abstract:
Adenosine triphosphate-binding cassette (ABC) transporters, such as multidrug resistance protein 1 (MRP1), protect against cellular toxicity by exporting xenobiotic compounds across the plasma membrane. However, constitutive MRP1 function hinders drug delivery across the blood-brain barrier, and MRP1 overexpression in certain cancers leads to acquired multidrug resistance and chemotherapy failure. Small-molecule inhibitors have the potential to block substrate transport, but few show specificity for MRP1. Here we identify a macrocyclic peptide, named CPI1, which inhibits MRP1 with nanomolar potency but shows minimal inhibition of a related multidrug transporter P-glycoprotein. A cryoelectron microscopy (cryo-EM) structure at 3.27 Å resolution shows that CPI1 binds MRP1 at the same location as the physiological substrate leukotriene C4 (LTC 4 ). Residues that interact with both ligands contain large, flexible sidechains that can form a variety of interactions, revealing how MRP1 recognizes multiple structurally unrelated molecules. CPI1 binding prevents the conformational changes necessary for adenosine triphosphate (ATP) hydrolysis and substrate transport, suggesting it may have potential as a therapeutic candidate.
Organizational Affiliation:
Laboratory of Membrane Biology and Biophysics, The Rockefeller University, New York, NY 10065.