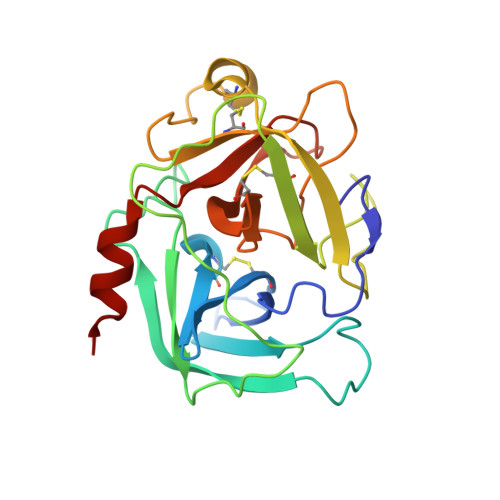
The structure of the pro-apoptotic protease granzyme B reveals the molecular determinants of its specificity
Waugh, S.M., Harris, J.L., Fletterick, R., Craik, C.S.(2000) Nat Struct Biol 7: 762-765
- PubMed: 10966646
- DOI: https://doi.org/10.1038/78992
- Primary Citation of Related Structures:
1FI8 - PubMed Abstract:
Granzyme B is a serine protease of the chymotrypsin fold that mediates cell death by cytotoxic lymphocytes. It is a processing enzyme, requiring extended peptide substrates containing an Asp residue. The determinants that allow for this substrate specificity are revealed in the three-dimensional structure of granzyme B in complex with a macromolecular inhibitor. The primary specificity for Asp occurs through a side-on interaction with Arg 226, a buried Arg side chain of granzyme B. An additional nine amino acids make contact with the substrate and define the granzyme B extended substrate specificity profile. The substrate determinants found in this structure are shared by other members of this protein class and help to reveal the properties that define substrate specificity.
- The Graduate Group in Biophysics, University of California, San Francisco, California 94143-0446, USA. waugh@mutant.ucsf.edu
Organizational Affiliation: