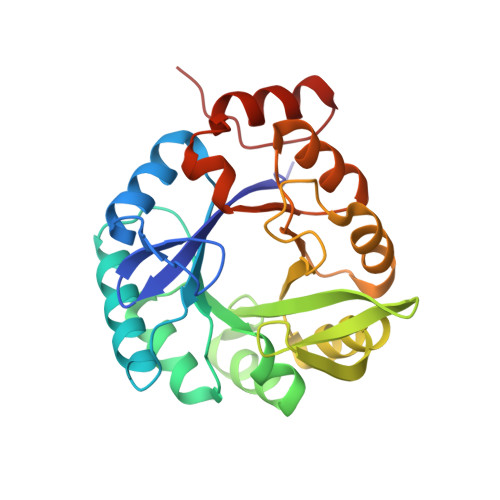
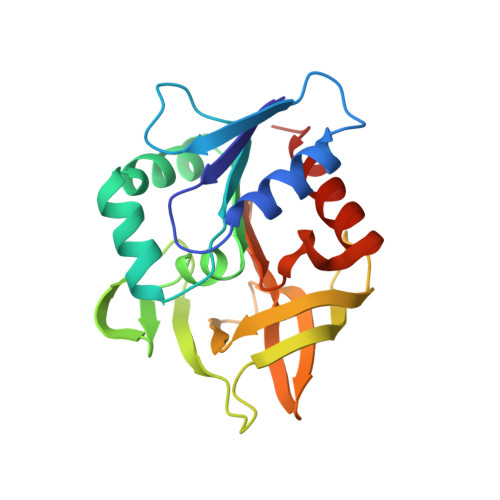
Structural Evidence for Ammonia Tunneling Across the (Beta Alpha)(8) Barrel of the Imidazole Glycerol Phosphate Synthase Bienzyme Complex.
Douangamath, A., Walker, M., Beismann-Driemeyer, S., Vega-Fernandez, M.C., Sterner, R., Wilmanns, M.(2002) Structure 10: 185
- PubMed: 11839304
- DOI: https://doi.org/10.1016/s0969-2126(02)00702-5
- Primary Citation of Related Structures:
1GPW, 1K9V - PubMed Abstract:
Since reactive ammonia is not available under physiological conditions, glutamine is used as a source for the incorporation of nitrogen in a number of metabolic pathway intermediates. The heterodimeric ImGP synthase that links histidine and purine biosynthesis belongs to the family of glutamine amidotransferases in which the glutaminase activity is coupled with a subsequent synthase activity specific for each member of the enzyme family. Its X-ray structure from the hyperthermophile Thermotoga maritima shows that the glutaminase subunit is associated with the N-terminal face of the (beta alpha)(8) barrel cyclase subunit. The complex reveals a putative tunnel for the transfer of ammonia over a distance of 25 A. Although ammonia tunneling has been reported for glutamine amidotransferases, the ImGP synthase has evolved a novel mechanism, which extends the known functional properties of the versatile (beta alpha)(8) barrel fold.
- EMBL Hamburg Outstation c/o DESY, Notkestrasse 85, D-22603 Hamburg, Germany.
Organizational Affiliation: