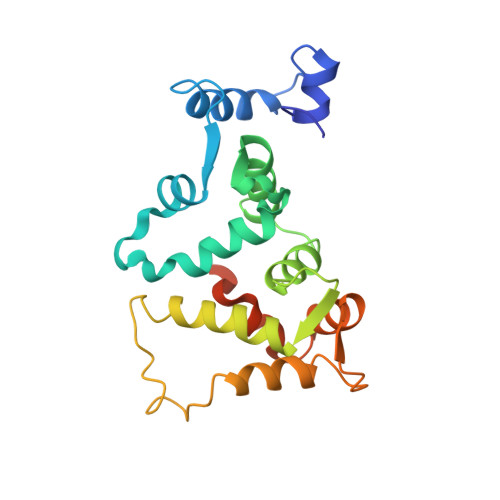
Three-dimensional structure of guanylyl cyclase activating protein-2, a calcium-sensitive modulator of photoreceptor guanylyl cyclases.
Ames, J.B., Dizhoor, A.M., Ikura, M., Palczewski, K., Stryer, L.(1999) J Biological Chem 274: 19329-19337
- PubMed: 10383444
- DOI: https://doi.org/10.1074/jbc.274.27.19329
- Primary Citation of Related Structures:
1JBA - PubMed Abstract:
Guanylyl cyclase activating protein-2 (GCAP-2) is a Ca2+-sensitive regulator of phototransduction in retinal photoreceptor cells. GCAP-2 activates retinal guanylyl cyclases at low Ca2+ concentration (<100 nM) and inhibits them at high Ca2+ (>500 nM). The light-induced lowering of the Ca2+ level from approximately 500 nM in the dark to approximately 50 nM following illumination is known to play a key role in visual recovery and adaptation. We report here the three-dimensional structure of unmyristoylated GCAP-2 with three bound Ca2+ ions as determined by nuclear magnetic resonance spectroscopy of recombinant, isotopically labeled protein. GCAP-2 contains four EF-hand motifs arranged in a compact tandem array like that seen previously in recoverin. The root mean square deviation of the main chain atoms in the EF-hand regions is 2.2 A in comparing the Ca2+-bound structures of GCAP-2 and recoverin. EF-1, as in recoverin, does not bind calcium because it contains a disabling Cys-Pro sequence. GCAP-2 differs from recoverin in that the calcium ion binds to EF-4 in addition to EF-2 and EF-3. A prominent exposed patch of hydrophobic residues formed by EF-1 and EF-2 (Leu24, Trp27, Phe31, Phe45, Phe48, Phe49, Tyr81, Val82, Leu85, and Leu89) may serve as a target-binding site for the transmission of calcium signals to guanylyl cyclase.
- Center for Advanced Research in Biotechnology, University of Maryland Biotechnology Institute, Rockville, Maryland 20850, USA. james@carb.nist.gov
Organizational Affiliation: