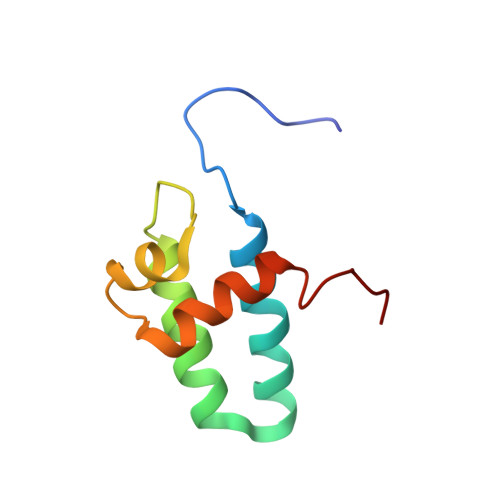
Solution Structure of the Lyase Domain of Human DNA Polymerase Lambda
DeRose, E.F., Kirby, T.W., Mueller, G.A., Bebenek, K., Garcia-Diaz, M., Blanco, L., Kunkel, T.A., London, R.E.(2003) Biochemistry 42: 9564-9574
- PubMed: 12911298
- DOI: https://doi.org/10.1021/bi034298s
- Primary Citation of Related Structures:
1NZP - PubMed Abstract:
DNA polymerase lambda (pol lambda) is a recently discovered nuclear enzyme belonging to the pol X family of DNA polymerases that exhibits a 32% sequence identity with the nuclear DNA repair protein, pol beta. Structural modeling suggests that pol lambda contains the palm, fingers, thumb, and 8 kDa lyase domains present in pol beta, as well as an additional N-terminal BRCT domain and a serine-proline-rich linker that are presumably involved in protein-protein interactions. The 8 kDa domain of pol beta is important for DNA binding and contains the dRP lyase activity, which is the rate-limiting step in the single-nucleotide base excision repair (BER) pathway of damaged DNA. Recently, it was shown that the 8 kDa domain of pol lambda also contains the dRP lyase activity. To gain further insight into the catalytic mechanism of dRP removal by pol lambda, we have determined the solution structure of the 8 kDa lyase domain of human DNA pol lambda via multidimensional NMR methods and the ARIA program. The resulting structures exhibited a high degree of similarity with the 8 kDa lyase domain of pol beta. Specifically, the side chains of residues W274, R275, Y279, K307, R308, and K312 are in similar positions to the functionally important side chains of residues H34, K35, Y39, K60, K68, and K72 in the 8 kDa lyase domain of pol beta. This suggests that, on the basis of the proposed roles of these residues in pol beta, the corresponding pol lambda side chains may be involved in DNA binding and dRP lyase activity. The structural alignment of W274 (pol lambda) with H34 (pol beta) indicates that the former is probably involved in a similar base stacking interaction with template DNA at the position of the gap, in contrast with several previous proposals which aligned D272 with H34. In a few cases for which there is a nonconservative substitution in the sequence alignment, a structural comparison shows a positionally and, hence, probably a functionally equivalent residue, e.g., K60 in pol beta and K307 in pol lambda. Additionally, on the basis of the structural alignment obtained, several previously proposed mechanistic hypotheses can be evaluated.
- Laboratory of Structural Biology, National Institute of Environmental Health Sciences, National Institutes of Health, Box 12233, Research Triangle Park, North Carolina 27709, USA.
Organizational Affiliation: