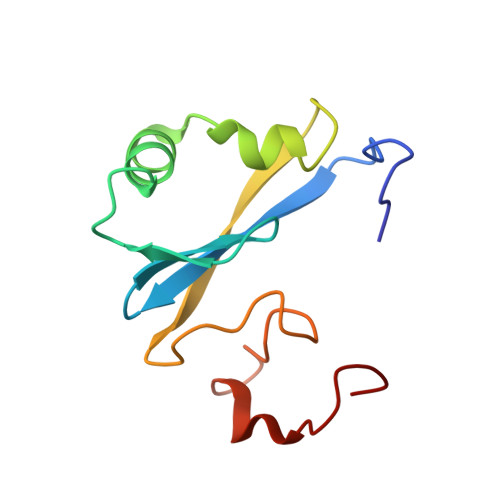
The SEP domain of p47 acts as a reversible competitive inhibitor of cathepsin L
Soukenik, M., Diehl, A., Leidert, M., Sievert, V., Buessow, K., Leitner, D., Labudde, D., Ball, L.J., Lechner, A., Nagler, D.K., Oschkinat, H.(2004) FEBS Lett 576: 358-362
- PubMed: 15498563
- DOI: https://doi.org/10.1016/j.febslet.2004.09.037
- Primary Citation of Related Structures:
1SS6 - PubMed Abstract:
The solution structure of the human p47 SEP domain in a construct comprising residues G1-S2-p47(171-270) was determined by NMR spectroscopy. A structure-derived hypothesis about the domains' function was formulated and pursued in binding experiments with cysteine proteases. The SEP domain was found to be a reversible competitive inhibitor of cathepsin L with a Ki of 1.5 microM. The binding of G1-S2-p47(171-270) to cathepsin L was mapped by biochemical assays and the binding interface was investigated by NMR chemical shift perturbation experiments.
- Forschungsinstitut für Molekulare Pharmakologie, Robert-Rössle Str. 10, D-13125 Berlin, Germany.
Organizational Affiliation: