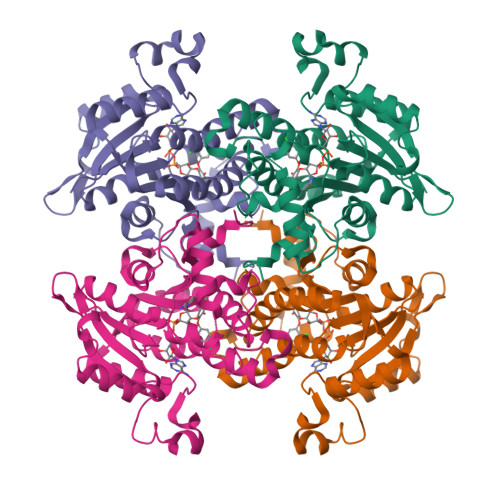
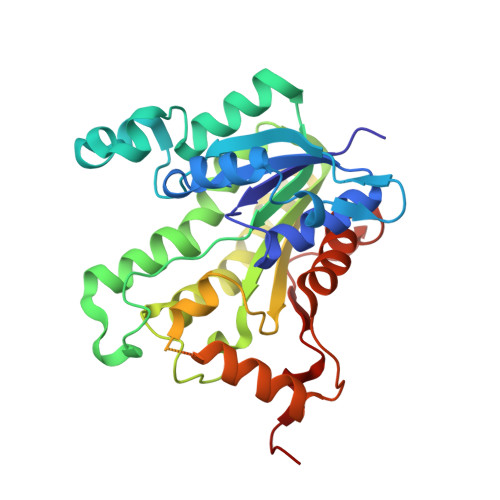
Structural basis for the variation in triclosan affinity to enoyl reductases.
Pidugu, L.S., Kapoor, M., Surolia, N., Surolia, A., Suguna, K.(2004) J Mol Biol 343: 147-155
- PubMed: 15381426
- DOI: https://doi.org/10.1016/j.jmb.2004.08.033
- Primary Citation of Related Structures:
1UH5, 1V35 - PubMed Abstract:
Bacteria synthesize fatty acids in a dissociated type pathway different from that in humans. Enoyl acyl carrier protein reductase, which catalyzes the final step of fatty acid elongation, has been validated as a potential anti-microbial drug target. Triclosan is known to inhibit this enzyme effectively. Precise characterization of the mode of triclosan binding is required to develop highly specific inhibitors. With this in view, interactions between triclosan, the cofactor NADH/NAD+ and the enzyme from five different species, one plant and four of microbial origin, have been examined in the available crystal structures. A comparison of these structures shows major structural differences at the substrate/inhibitor/cofactor-binding loop. The analysis reveals that the conformation of this flexible loop and the binding affinities of triclosan to each of these enzymes are strongly correlated.
Organizational Affiliation:
Molecular Biophysics Unit, Indian Institute of Science, Bangalore 560 012.