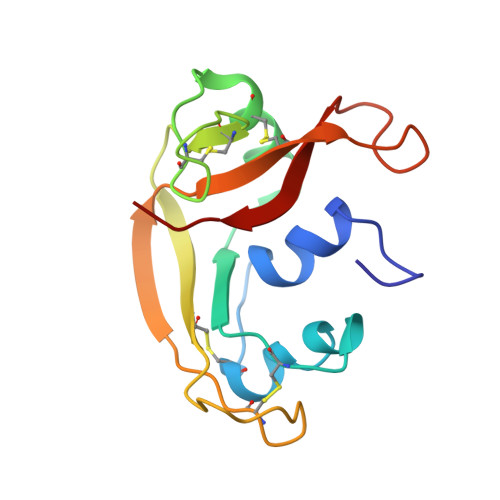
The (1)H, (13)C, (15)N resonance assignment, solution structure, and residue level stability of eosinophil cationic protein/RNase 3 determined by NMR spectroscopy
Laurents, D.V., Bruix, M., Jimenez, M.A., Santoro, J., Boix, E., Moussaoui, M., Nogues, M.V., Rico, M.(2009) Biopolymers 91: 1018-1028
- PubMed: 19189375
- DOI: https://doi.org/10.1002/bip.21152
- Primary Citation of Related Structures:
2KB5 - PubMed Abstract:
Eosinophil cationic protein (ECP)/human RNase 3, a member of the RNase A family, is a remarkably cytotoxic protein implicated in asthma and allergies. These activities are probably due to ECP's ability to interact with and disrupt membranes and depend on two Trp, 19 Arg, and possibly an extremely high conformational stability. Here, we have used NMR spectroscopy to assign essentially all (1)H, (15)N, and backbone (13)C resonances, to solve the 3D structure in aqueous solution and to quantify its residue-level stability. The NMR solution structure was determined on the basis of 2316 distance constraints and is well-defined (backbone RMSD = 0.81 A). The N-terminus and the loop composed of residues 114-123 are relatively well-ordered; in contrast, conformational diversity is observed for the loop segments 17-22, 65-68, and 92-95 and most exposed sidechains. The side chain NH groups of the two Trp and 19 Arg showed no significant protection against hydrogen/deuterium exchange. The most protected NH groups belong to the first and last two beta-strands, and curiously, the first alpha-helix. Analysis of their exchange rates reveals a strikingly high global stability of 11.8 kcal/mol. This value and other stability measurements are used to better quantify ECP's unfolding thermodynamics.
- Instituto de Química Física "Rocasolano", CSIC, Madrid, Spain.
Organizational Affiliation: