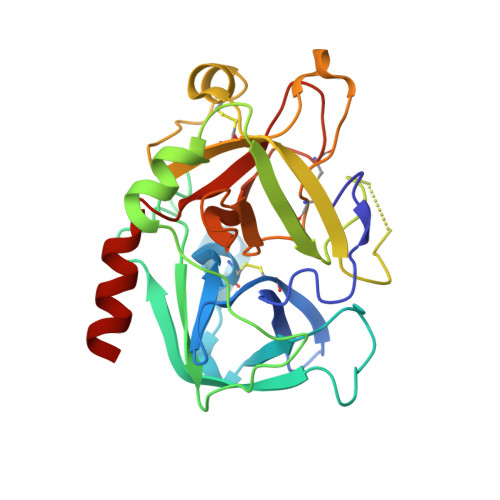
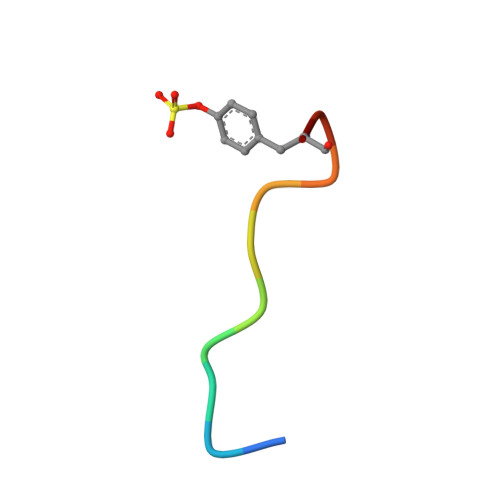
Non-additivity of functional group contributions in protein-ligand binding: a comprehensive study by crystallography and isothermal titration calorimetry.
Baum, B., Muley, L., Smolinski, M., Heine, A., Hangauer, D., Klebe, G.(2010) J Mol Biology 397: 1042-1054
- PubMed: 20156458
- DOI: https://doi.org/10.1016/j.jmb.2010.02.007
- Primary Citation of Related Structures:
2ZGB, 2ZHQ, 2ZI2, 2ZNK - PubMed Abstract:
Additivity of functional group contributions to protein-ligand binding is a very popular concept in medicinal chemistry as the basis of rational design and optimized lead structures. Most of the currently applied scoring functions for docking build on such additivity models. Even though the limitation of this concept is well known, case studies examining in detail why additivity fails at the molecular level are still very scarce. The present study shows, by use of crystal structure analysis and isothermal titration calorimetry for a congeneric series of thrombin inhibitors, that extensive cooperative effects between hydrophobic contacts and hydrogen bond formation are intimately coupled via dynamic properties of the formed complexes. The formation of optimal lipophilic contacts with the surface of the thrombin S3 pocket and the full desolvation of this pocket can conflict with the formation of an optimal hydrogen bond between ligand and protein. The mutual contributions of the competing interactions depend on the size of the ligand hydrophobic substituent and influence the residual mobility of ligand portions at the binding site. Analysis of the individual crystal structures and factorizing the free energy into enthalpy and entropy demonstrates that binding affinity of the ligands results from a mixture of enthalpic contributions from hydrogen bonding and hydrophobic contacts, and entropic considerations involving an increasing loss of residual mobility of the bound ligands. This complex picture of mutually competing and partially compensating enthalpic and entropic effects determines the non-additivity of free energy contributions to ligand binding at the molecular level.
- Department of Pharmaceutical Chemistry, Philipps-University Marburg, Marbacher Weg 6, 35032 Marburg, Germany.
Organizational Affiliation: