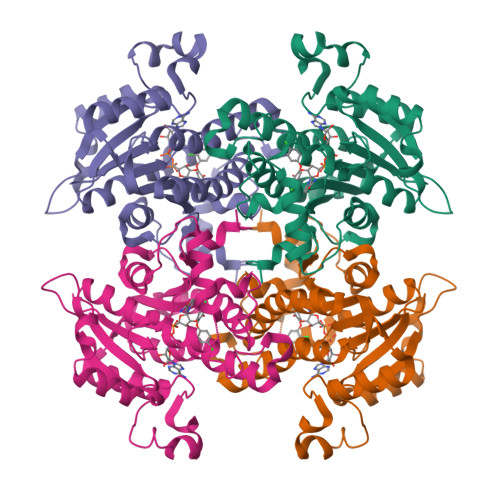
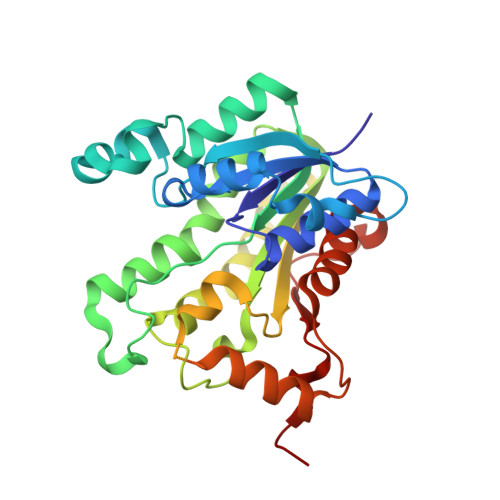
Effect of substrate binding loop mutations on the structure, kinetics, and inhibition of enoyl acyl carrier protein reductase from plasmodium falciparum
Maity, K., Banerjee, T., Prabakaran, N., Surolia, N., Surolia, A., Suguna, K.(2011) IUBMB Life 63: 30-41
- PubMed: 21280175
- DOI: https://doi.org/10.1002/iub.412
- Primary Citation of Related Structures:
3AM3, 3AM4, 3AM5 - PubMed Abstract:
Enoyl acyl carrier protein reductase (ENR), which catalyzes the final and rate limiting step of fatty acid elongation, has been validated as a potential drug target. Triclosan is known to be an effective inhibitor for this enzyme. We mutated the substrate binding site residue Ala372 of the ENR of Plasmodium falciparum (PfENR) to Methionine and Valine which increased the affinity of the enzyme towards triclosan to almost double, close to that of Escherichia coli ENR (EcENR) which has a Methionine at the structurally similar position of Ala372 of PfENR. Kinetic studies of the mutants of PfENR and the crystal structure analysis of the A372M mutant revealed that a more hydrophobic environment enhances the affinity of the enzyme for the inhibitor. A triclosan derivative showed a threefold increase in the affinity towards the mutants compared to the wild type, due to additional interactions with the A372M mutant as revealed by the crystal structure. The enzyme has a conserved salt bridge which stabilizes the substrate binding loop and appears to be important for the active conformation of the enzyme. We generated a second set of mutants to check this hypothesis. These mutants showed loss of function, except in one case, where the crystal structure showed that the substrate binding loop is stabilized by a water bridge network.
Organizational Affiliation:
Molecular Biophysics Unit, Indian Institute of Science, Bangalore, India.