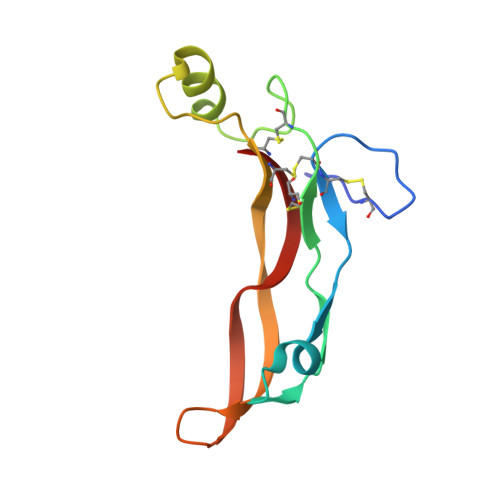
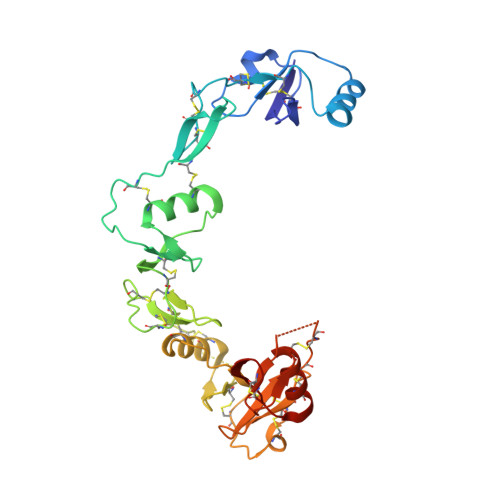
The structure of myostatin:follistatin 288: insights into receptor utilization and heparin binding
Cash, J.N., Rejon, C.A., McPherron, A.C., Bernard, D.J., Thompson, T.B.(2009) EMBO J 28: 2662-2676
- PubMed: 19644449
- DOI: https://doi.org/10.1038/emboj.2009.205
- Primary Citation of Related Structures:
3HH2 - PubMed Abstract:
Myostatin is a member of the transforming growth factor-beta (TGF-beta) family and a strong negative regulator of muscle growth. Here, we present the crystal structure of myostatin in complex with the antagonist follistatin 288 (Fst288). We find that the prehelix region of myostatin very closely resembles that of TGF-beta class members and that this region alone can be swapped into activin A to confer signalling through the non-canonical type I receptor Alk5. Furthermore, the N-terminal domain of Fst288 undergoes conformational rearrangements to bind myostatin and likely acts as a site of specificity for the antagonist. In addition, a unique continuous electropositive surface is created when myostatin binds Fst288, which significantly increases the affinity for heparin. This translates into stronger interactions with the cell surface and enhanced myostatin degradation in the presence of either Fst288 or Fst315. Overall, we have identified several characteristics unique to myostatin that will be paramount to the rational design of myostatin inhibitors that could be used in the treatment of muscle-wasting disorders.
- Department of Molecular Genetics, Biochemistry, and Microbiology, University of Cincinnati, Cincinnati, OH 45267, USA.
Organizational Affiliation: