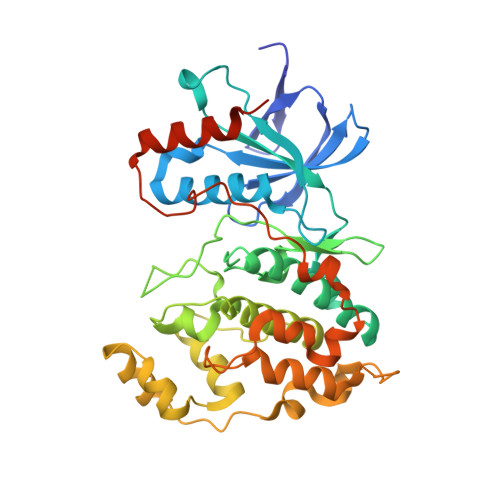
Structural and Functional Analysis of the Natural JNK1 Inhibitor Quercetagetin.
Baek, S., Kang, N.J., Popowicz, G.M., Arciniega, M., Jung, S.K., Byun, S., Song, N.R., Heo, Y.S., Kim, B.Y., Lee, H.J., Holak, T.A., Augustin, M., Bode, A.M., Huber, R., Dong, Z., Lee, K.W.(2013) J Mol Biology 425: 411-423
- PubMed: 23142567
- DOI: https://doi.org/10.1016/j.jmb.2012.10.019
- Primary Citation of Related Structures:
3V3V - PubMed Abstract:
c-Jun NH2-terminal kinases (JNKs) and phosphatidylinositol 3-kinase (PI3-K) play critical roles in chronic diseases such as cancer, type II diabetes, and obesity. We describe here the binding of quercetagetin (3,3',4',5,6,7-hydroxyflavone), related flavonoids, and SP600125 to JNK1 and PI3-K by ATP-competitive and immobilized metal ion affinity-based fluorescence polarization assays and measure the effect of quercetagetin on JNK1 and PI3-K activities. Quercetagetin attenuated the phosphorylation of c-Jun and AKT, suppressed AP-1 and NF-κB promoter activities, and also reduced cell transformation. It attenuated tumor incidence and reduced tumor volumes in a two-stage skin carcinogenesis mouse model. Our crystallographic structure determination data show that quercetagetin binds to the ATP-binding site of JNK1. Notably, the interaction between Lys55, Asp169, and Glu73 of JNK1 and the catechol moiety of quercetagetin reorients the N-terminal lobe of JNK1, thereby improving compatibility of the ligand with its binding site. The results of a theoretical docking study suggest a binding mode of PI3-K with the hydroxyl groups of the catechol moiety forming hydrogen bonds with the side chains of Asp964 and Asp841 in the p110γ catalytic subunit. These interactions could contribute to the high inhibitory activity of quercetagetin against PI3-K. Our study suggests the potential use of quercetagetin in the prevention or therapy of cancer and other chronic diseases.
- Max Planck Institute for Biochemistry, Am Klopferspitz 18, 82152 Martinsried, Germany.
Organizational Affiliation: