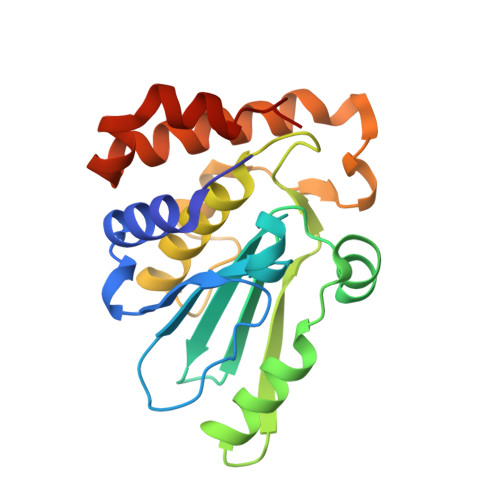
Structure-based design and optimization of potent inhibitors of the adenoviral protease.
Grosche, P., Sirockin, F., Mac Sweeney, A., Ramage, P., Erbel, P., Melkko, S., Bernardi, A., Hughes, N., Ellis, D., Combrink, K.D., Jarousse, N., Altmann, E.(2015) Bioorg Med Chem Lett 25: 438-443
- PubMed: 25571794
- DOI: https://doi.org/10.1016/j.bmcl.2014.12.057
- Primary Citation of Related Structures:
4WX4, 4WX6, 4WX7 - PubMed Abstract:
Adenoviral infections are associated with a wide range of acute diseases, among which ocular viral conjunctivitis (EKC) and disseminated disease in immunocompromised patients. To date, no approved specific anti-adenoviral drug is available, but there is a growing need for an effective treatment of such infections. The adenoviral protease, adenain, plays a crucial role for the viral lifecycle and thus represents an attractive therapeutic target. Structure-guided design with the objective to depeptidize tetrapeptide nitrile 1 led to the novel chemotype 2. Optimization of scaffold 2 resulted in picomolar adenain inhibitors 3a and 3b. In addition, a complementary series of irreversible vinyl sulfone containing inhibitors were rationally designed, prepared and evaluated against adenoviral protease. High resolution X-ray co-crystal structures of representatives of each series proves the successful design of these inhibitors and provides an excellent basis for future medicinal chemistry optimization of these compounds.
Organizational Affiliation:
Novartis Institute for Biomedical Research, Novartis Campus, CH-4002 Basel, Switzerland.