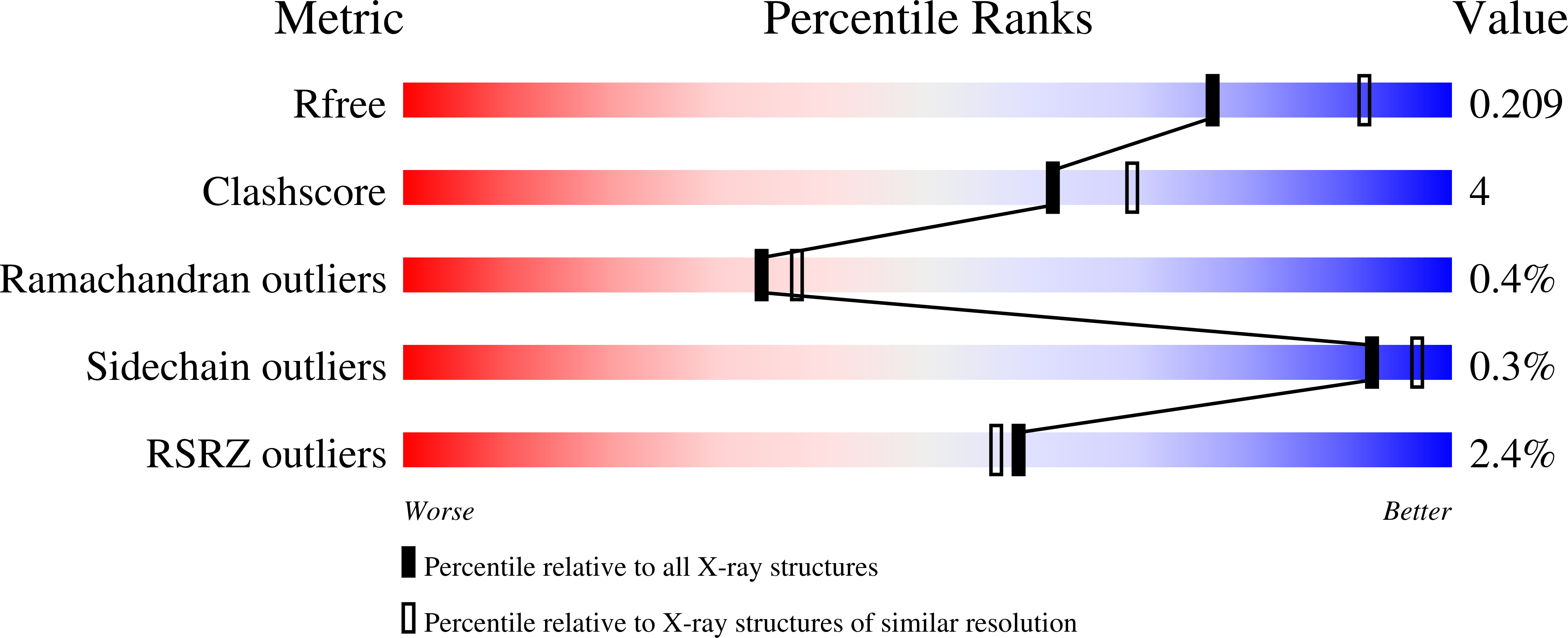
Mechanism of inactivation of gamma-aminobutyric acid aminotransferase by (1S,3S)-3-amino-4-difluoromethylenyl-1-cyclopentanoic acid (CPP-115)
Hyunbeom, L., Emma, H.D., Rui, W., Ruslan, S., Jose, I.J., Dali, L., Neil, K., Richard, B.S.(2015) J Am Chem Soc
- PubMed: 25616005
- DOI: https://doi.org/10.1021/ja512299n
- Primary Citation of Related Structures:
4Y0D, 4Y0H - PubMed Abstract:
γ-Aminobutyric acid aminotransferase (GABA-AT) is a pyridoxal 5'-phosphate (PLP)-dependent enzyme that degrades GABA, the principal inhibitory neurotransmitter in mammalian cells. When the concentration of GABA falls below a threshold level, convulsions can occur. Inhibition of GABA-AT raises GABA levels in the brain, which can terminate seizures as well as have potential therapeutic applications in treating other neurological disorders, including drug addiction. Among the analogues that we previously developed, (1S,3S)-3-amino-4-difluoromethylene-1-cyclopentanoic acid (CPP-115) showed 187 times greater potency than that of vigabatrin, a known inactivator of GABA-AT and approved drug (Sabril) for the treatment of infantile spasms and refractory adult epilepsy. Recently, CPP-115 was shown to have no adverse effects in a Phase I clinical trial. Here we report a novel inactivation mechanism for CPP-115, a mechanism-based inactivator that undergoes GABA-AT-catalyzed hydrolysis of the difluoromethylene group to a carboxylic acid with concomitant loss of two fluoride ions and coenzyme conversion to pyridoxamine 5'-phosphate (PMP). The partition ratio for CPP-115 with GABA-AT is about 2000, releasing cyclopentanone-2,4-dicarboxylate (22) and two other precursors of this compound (20 and 21). Time-dependent inactivation occurs by a conformational change induced by the formation of the aldimine of 4-aminocyclopentane-1,3-dicarboxylic acid and PMP (20), which disrupts an electrostatic interaction between Glu270 and Arg445 to form an electrostatic interaction between Arg445 and the newly formed carboxylate produced by hydrolysis of the difluoromethylene group in CPP-115, resulting in a noncovalent, tightly bound complex. This represents a novel mechanism for inactivation of GABA-AT and a new approach for the design of mechanism-based inactivators in general.
Organizational Affiliation:
Department of Chemistry, Chemistry of Life Processes Institute, and Center for Molecular Innovation and Drug Discovery, Northwestern University , 2145 Sheridan Road, Evanston, Illinois 60208-3113, United States.