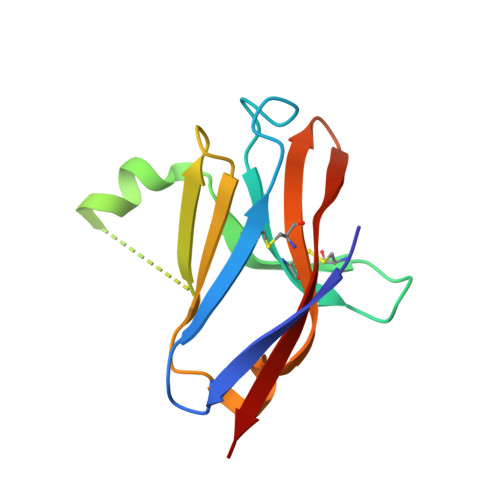
Molecular basis for the loss-of-function effects of the Alzheimer's disease-associated R47H variant of the immune receptor TREM2.
Sudom, A., Talreja, S., Danao, J., Bragg, E., Kegel, R., Min, X., Richardson, J., Zhang, Z., Sharkov, N., Marcora, E., Thibault, S., Bradley, J., Wood, S., Lim, A.C., Chen, H., Wang, S., Foltz, I.N., Sambashivan, S., Wang, Z.(2018) J Biological Chem 293: 12634-12646
- PubMed: 29794134
- DOI: https://doi.org/10.1074/jbc.RA118.002352
- Primary Citation of Related Structures:
5UD7, 5UD8, 6B8O - PubMed Abstract:
Triggering receptor expressed on myeloid cells 2 (TREM2) is an immune receptor expressed on the surface of microglia, macrophages, dendritic cells, and osteoclasts. The R47H TREM2 variant is a significant risk factor for late-onset Alzheimer's disease (AD), and the molecular basis of R47H TREM2 loss of function is an emerging area of TREM2 biology. Here, we report three high-resolution structures of the extracellular ligand-binding domains (ECDs) of R47H TREM2, apo-WT, and phosphatidylserine (PS)-bound WT TREM2 at 1.8, 2.2, and 2.2 Å, respectively. The structures reveal that Arg 47 plays a critical role in maintaining the structural features of the complementarity-determining region 2 (CDR2) loop and the putative positive ligand-interacting surface (PLIS), stabilizing conformations capable of ligand interaction. This is exemplified in the PS-bound structure, in which the CDR2 loop and PLIS drive critical interactions with PS via surfaces that are disrupted in the variant. Together with in vitro and in vivo characterization, our structural findings elucidate the molecular mechanism underlying loss of ligand binding, putative oligomerization, and functional activity of R47H TREM2. They also help unravel how decreased in vitro and in vivo stability of TREM2 contribute to loss of function in disease.
- From Amgen Discovery Research, Amgen Inc., San Francisco, California 94080, asudom@amgen.com.
Organizational Affiliation: