Leishmania donovani tyrosyl-tRNA synthetase structure in complex with a tyrosyl adenylate analog and comparisons with human and protozoan counterparts.
Barros-Alvarez, X., Kerchner, K.M., Koh, C.Y., Turley, S., Pardon, E., Steyaert, J., Ranade, R.M., Gillespie, J.R., Zhang, Z., Verlinde, C.L.M.J., Fan, E., Buckner, F.S., Hol, W.G.J.(2017) Biochimie 138: 124-136
- PubMed: 28427904
- DOI: https://doi.org/10.1016/j.biochi.2017.04.006
- Primary Citation of Related Structures:
5USF - PubMed Abstract:
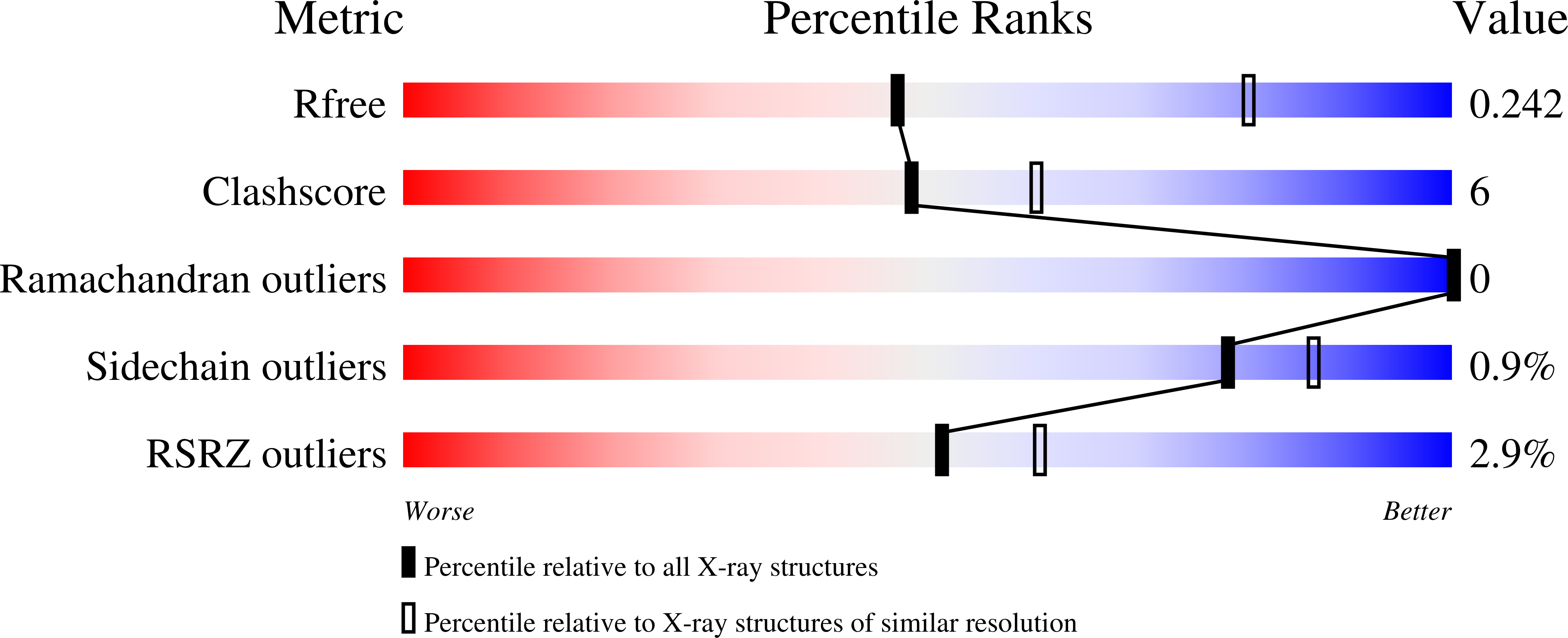
The crystal structure of Leishmania donovani tyrosyl-tRNA synthetase (LdTyrRS) in complex with a nanobody and the tyrosyl adenylate analog TyrSA was determined at 2.75 Å resolution. Nanobodies are the variable domains of camelid heavy chain-only antibodies. The nanobody makes numerous crystal contacts and in addition reduces the flexibility of a loop of LdTyrRS. TyrSA is engaged in many interactions with active site residues occupying the tyrosine and adenine binding pockets. The LdTyrRS polypeptide chain consists of two pseudo-monomers, each consisting of two domains. Comparing the two independent chains in the asymmetric unit reveals that the two pseudo-monomers of LdTyrRS can bend with respect to each other essentially as rigid bodies. This flexibility might be useful in the positioning of tRNA for catalysis since both pseudo-monomers in the LdTyrRS chain are needed for charging tRNA Tyr . An "extra pocket" (EP) appears to be present near the adenine binding region of LdTyrRS. Since this pocket is absent in the two human homologous enzymes, the EP provides interesting opportunities for obtaining selective drugs for treating infections caused by L. donovani, a unicellular parasite causing visceral leishmaniasis, or kala azar, which claims 20,000 to 30,000 deaths per year. Sequence and structural comparisons indicate that the EP is a characteristic which also occurs in the active site of several other important pathogenic protozoa. Therefore, the structure of LdTyrRS could inspire the design of compounds useful for treating several different parasitic diseases.
Organizational Affiliation:
Department of Biochemistry, University of Washington, Seattle, WA, USA; Laboratorio de Enzimología de Parásitos, Facultad de Ciencias, Universidad de los Andes, Mérida, Venezuela.