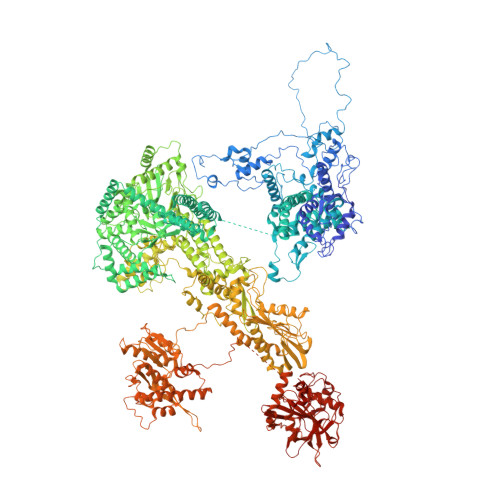
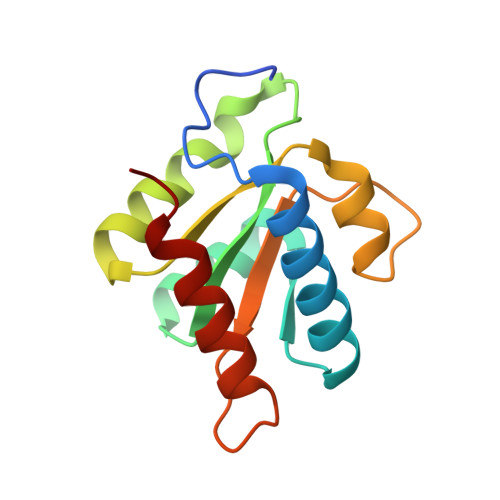
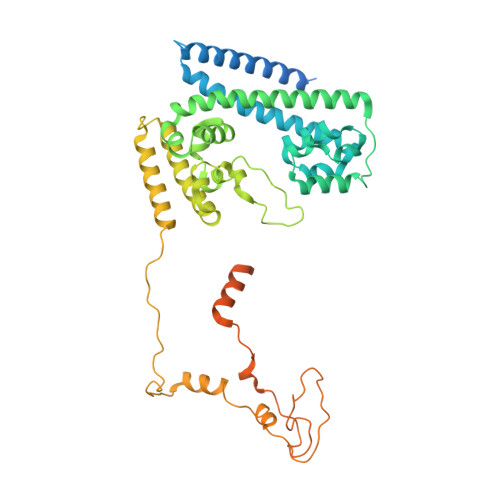
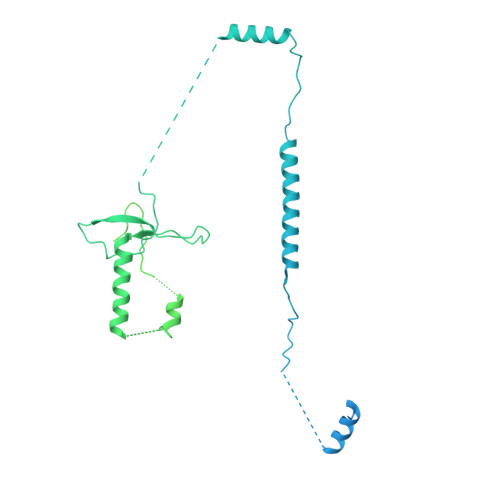
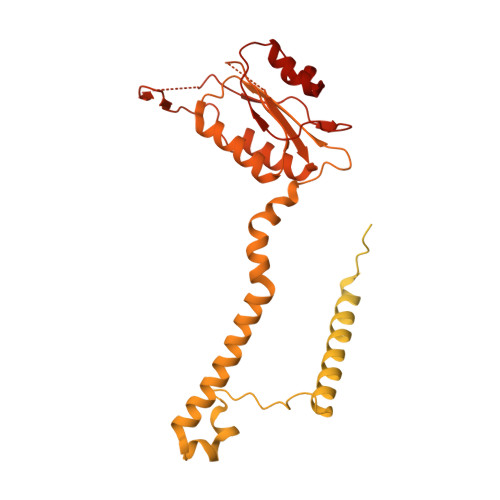
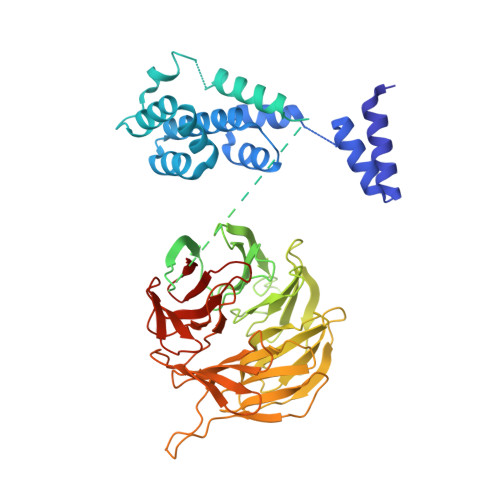
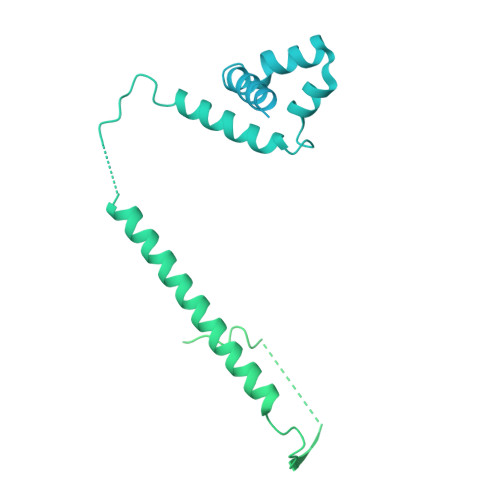
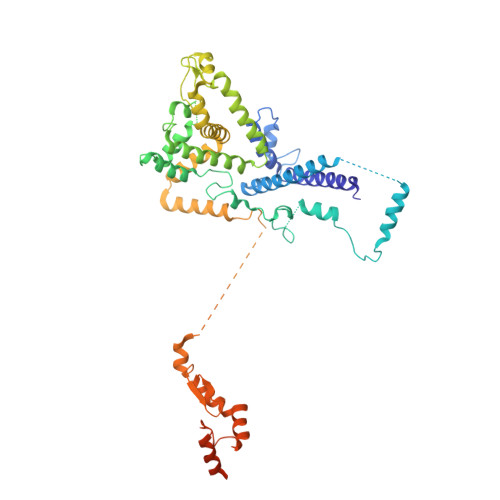
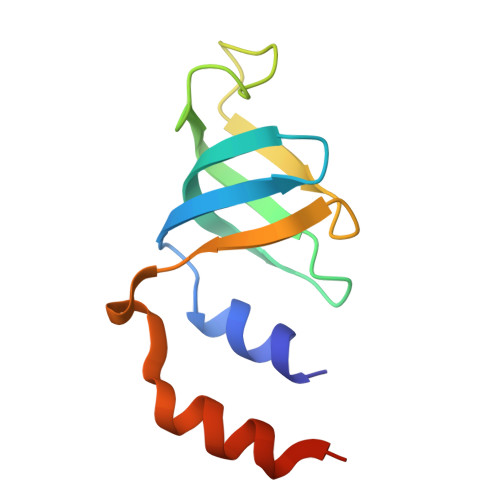
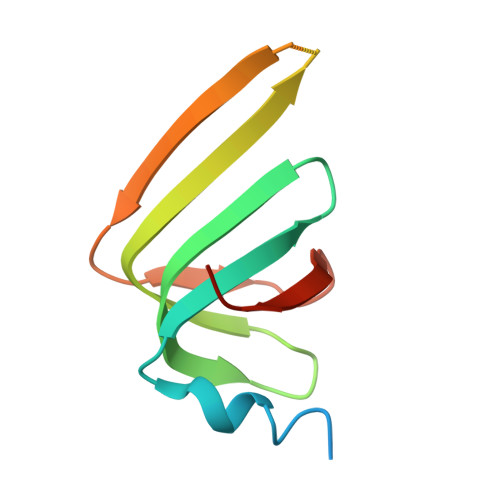
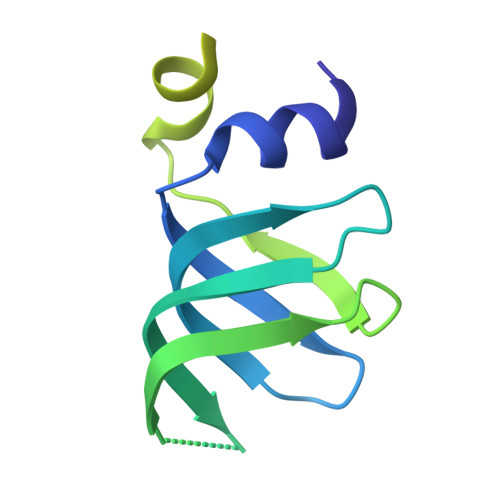
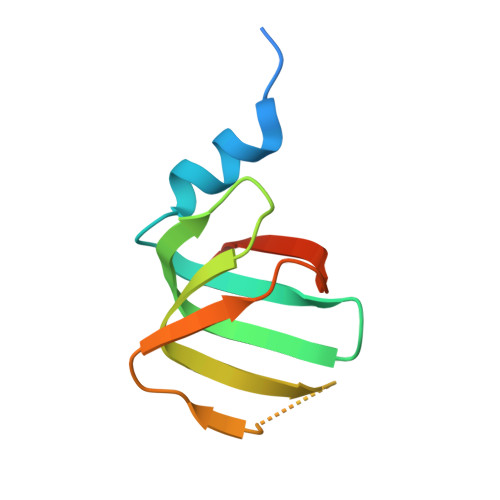
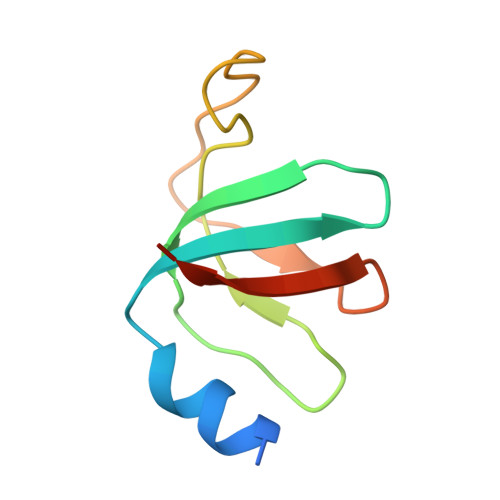
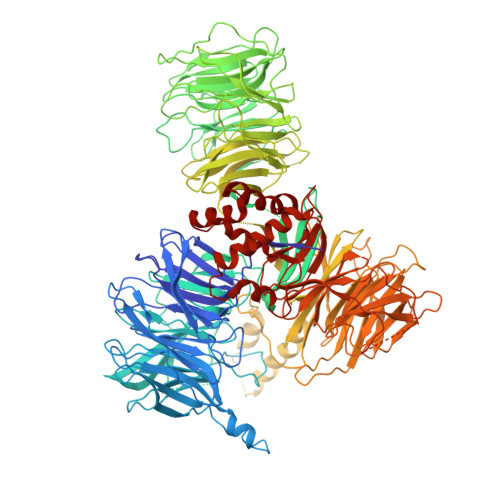
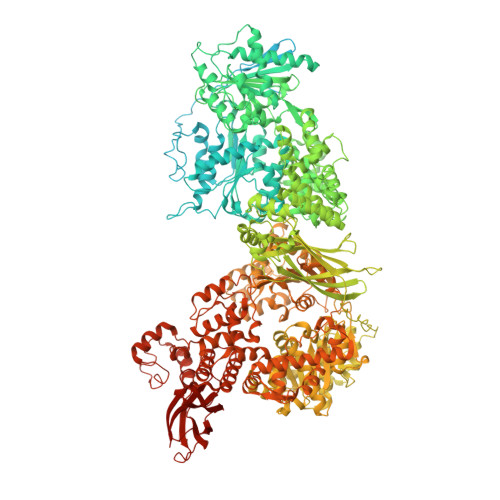
Structures of the human pre-catalytic spliceosome and its precursor spliceosome.
Zhan, X., Yan, C., Zhang, X., Lei, J., Shi, Y.(2018) Cell Res 28: 1129-1140
- PubMed: 30315277
- DOI: https://doi.org/10.1038/s41422-018-0094-7
- Primary Citation of Related Structures:
6AH0, 6AHD - PubMed Abstract:
The pre-catalytic spliceosome (B complex) is preceded by its precursor spliceosome (pre-B complex) and followed by the activated spliceosome (B act complex). The pre-B-to-B and B-to-B act transitions are driven by the ATPase/helicases Prp28 and Brr2, respectively. In this study, we report the cryo-electron microscopy structures of the human pre-B complex and the human B complex at an average resolution of 5.7 and 3.8 Å, respectively. In the pre-B complex, U1 and U2 small nuclear ribonucleoproteins (snRNPs) associate with two edges of the tetrahedron-shaped U4/U6.U5 tri-snRNP. The pre-mRNA is yet to be recognized by U5 or U6 small nuclear RNA (snRNA), and loop I of U5 snRNA remains unengaged. In the B complex, U1 snRNP and Prp28 are dissociated, the 5'-exon is anchored to loop I of U5 snRNA, and the 5'-splice site is recognized by U6 snRNA through duplex formation. In sharp contrast to S. cerevisiae, most components of U2 snRNP and tri-snRNP, exemplified by Brr2, undergo pronounced rearrangements in the human pre-B-to-B transition. Structural analysis reveals mechanistic insights into the assembly and activation of the human spliceosome.
- Beijing Advanced Innovation Center for Structural Biology, Tsinghua-Peking Joint Center for Life Sciences, School of Life Sciences and School of Medicine, Tsinghua University, Beijing, 100084, China.
Organizational Affiliation: