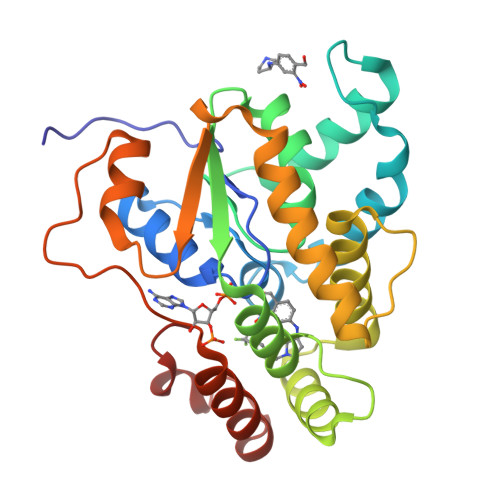
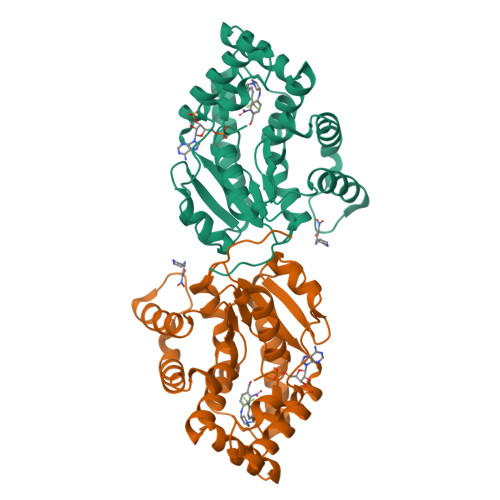
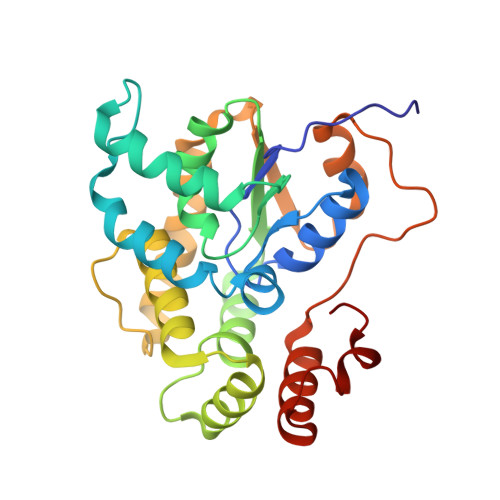
Design, Synthesis, and Characterization of Novel Small Molecules as Broad Range Antischistosomal Agents.
Rugel, A., Tarpley, R.S., Lopez, A., Menard, T., Guzman, M.A., Taylor, A.B., Cao, X., Kovalskyy, D., Chevalier, F.D., Anderson, T.J.C., Hart, P.J., LoVerde, P.T., McHardy, S.F.(2018) ACS Med Chem Lett 9: 967-973
- PubMed: 30344901
- DOI: https://doi.org/10.1021/acsmedchemlett.8b00257
- Primary Citation of Related Structures:
6BDP, 6BDQ, 6BDR, 6BDS, 6MFE - PubMed Abstract:
Schistosomiasis is a major human parasitic disease afflicting more than 250 million people, historically treated with chemotherapies praziquantel or oxamniquine. Since oxamniquine is species-specific, killing Schistosoma mansoni but not other schistosome species ( S. haematobium or S. japonicum ) and evidence for drug resistant strains is growing, research efforts have focused on identifying novel approaches. Guided by data from X-ray crystallographic studies and Schistosoma worm killing assays on oxamniquine, our structure-based drug design approach produced a robust structure-activity relationship (SAR) program that identified several new lead compounds with effective worm killing. These studies culminated in the discovery of compound 12a , which demonstrated broad-species activity in killing S. mansoni (75%), S. haematobium (40%), and S. japonicum (83%).
Organizational Affiliation:
Department of Pathology and Laboratory Medicine, Department of Biochemistry and Structural Biology, and Greehey Children's Cancer Research Institute, UT Health San Antonio, 7703 Floyd Curl Drive, San Antonio, Texas 78229, United States.