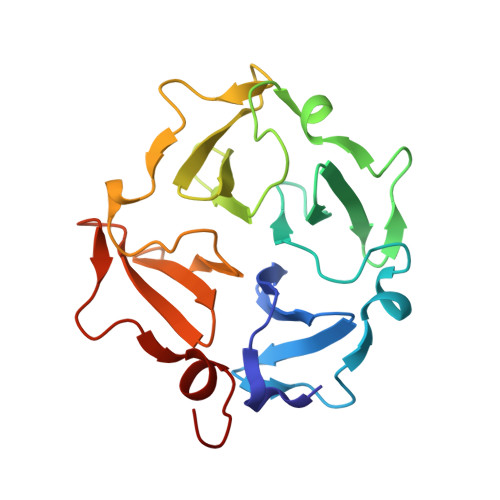
MT1-MMP Binds Membranes by Opposite Tips of Its beta Propeller to Position It for Pericellular Proteolysis.
Marcink, T.C., Simoncic, J.A., An, B., Knapinska, A.M., Fulcher, Y.G., Akkaladevi, N., Fields, G.B., Van Doren, S.R.(2019) Structure 27: 281-292.e6
- PubMed: 30471921
- DOI: https://doi.org/10.1016/j.str.2018.10.008
- Primary Citation of Related Structures:
6CLZ, 6CM1 - PubMed Abstract:
Critical to migration of tumor cells and endothelial cells is the proteolytic attack of membrane type 1 matrix metalloproteinase (MT1-MMP) upon collagen, growth factors, and receptors at cell surfaces. Lipid bilayer interactions of the substrate-binding hemopexin-like (HPX) domain of MT1-MMP were investigated by paramagnetic nuclear magnetic resonance relaxation enhancements (PREs), fluorescence, and mutagenesis. The HPX domain binds bilayers by blades II and IV on opposite sides of its β propeller fold. The EPGYPK sequence protruding from both blades inserts among phospholipid head groups in PRE-restrained molecular dynamics simulations. Bilayer binding to either blade II or IV exposes the CD44 binding site in blade I. Bilayer association with blade IV allows the collagen triple helix to bind without obstruction. Indeed, vesicles enhance proteolysis of collagen triple-helical substrates by the ectodomain of MT1-MMP. Hypothesized side-by-side MT1-MMP homodimerization would allow binding of bilayers, collagen, CD44, and head-to-tail oligomerization.
- Department of Biochemistry, University of Missouri, 117 Schweitzer Hall, Columbia, MO 65211, USA.
Organizational Affiliation: