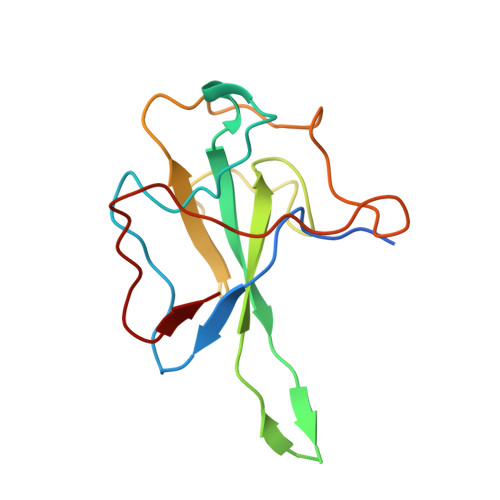
Crystal structure of SARS-CoV-2 nucleocapsid protein RNA binding domain reveals potential unique drug targeting sites.
Kang, S., Yang, M., Hong, Z., Zhang, L., Huang, Z., Chen, X., He, S., Zhou, Z., Zhou, Z., Chen, Q., Yan, Y., Zhang, C., Shan, H., Chen, S.(2020) Acta Pharm Sin B 10: 1228-1238
- PubMed: 32363136
- DOI: https://doi.org/10.1016/j.apsb.2020.04.009
- Primary Citation of Related Structures:
6M3M - PubMed Abstract:
The outbreak of coronavirus disease (COVID-19) caused by SARS-CoV-2 virus continually lead to worldwide human infections and deaths. Currently, there is no specific viral protein-targeted therapeutics. Viral nucleocapsid protein is a potential antiviral drug target, serving multiple critical functions during the viral life cycle. However, the structural information of SARS-CoV-2 nucleocapsid protein remains unclear. Herein, we have determined the 2.7 Å crystal structure of the N-terminal RNA binding domain of SARS-CoV-2 nucleocapsid protein. Although the overall structure is similar as other reported coronavirus nucleocapsid protein N-terminal domain, the surface electrostatic potential characteristics between them are distinct. Further comparison with mild virus type HCoV-OC43 equivalent domain demonstrates a unique potential RNA binding pocket alongside the β -sheet core. Complemented by in vitro binding studies, our data provide several atomic resolution features of SARS-CoV-2 nucleocapsid protein N-terminal domain, guiding the design of novel antiviral agents specific targeting to SARS-CoV-2.
- Molecular Imaging Center, Guangdong Provincial Key Laboratory of Biomedical Imaging, the Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai 519000, China.
Organizational Affiliation: