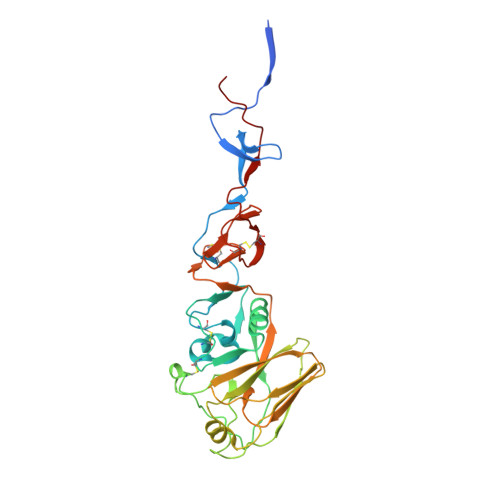
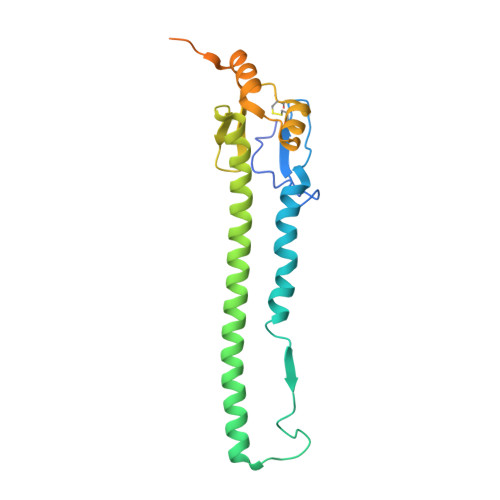
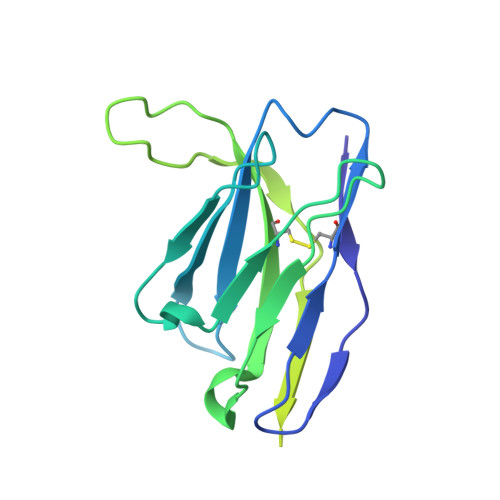
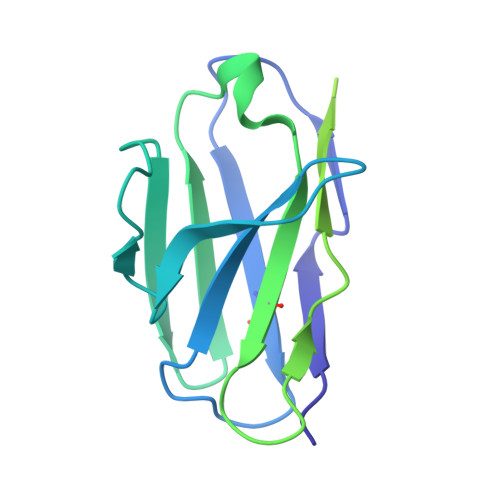
Potent anti-influenza H7 human monoclonal antibody induces separation of hemagglutinin receptor-binding head domains.
Turner, H.L., Pallesen, J., Lang, S., Bangaru, S., Urata, S., Li, S., Cottrell, C.A., Bowman, C.A., Crowe Jr., J.E., Wilson, I.A., Ward, A.B.(2019) PLoS Biol 17: e3000139-e3000139
- PubMed: 30716060
- DOI: https://doi.org/10.1371/journal.pbio.3000139
- Primary Citation of Related Structures:
6MLM - PubMed Abstract:
Seasonal influenza virus infections can cause significant morbidity and mortality, but the threat from the emergence of a new pandemic influenza strain might have potentially even more devastating consequences. As such, there is intense interest in isolating and characterizing potent neutralizing antibodies that target the hemagglutinin (HA) viral surface glycoprotein. Here, we use cryo-electron microscopy (cryoEM) to decipher the mechanism of action of a potent HA head-directed monoclonal antibody (mAb) bound to an influenza H7 HA. The epitope of the antibody is not solvent accessible in the compact, prefusion conformation that typifies all HA structures to date. Instead, the antibody binds between HA head protomers to an epitope that must be partly or transiently exposed in the prefusion conformation. The "breathing" of the HA protomers is implied by the exposure of this epitope, which is consistent with metastability of class I fusion proteins. This structure likely therefore represents an early structural intermediate in the viral fusion process. Understanding the extent of transient exposure of conserved neutralizing epitopes also may lead to new opportunities to combat influenza that have not been appreciated previously.
- Department of Integrative Structural and Computational Biology, The Scripps Research Institute, La Jolla, California, United States of America.
Organizational Affiliation: