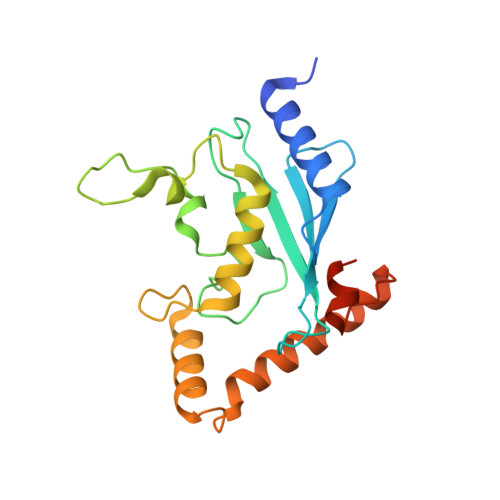
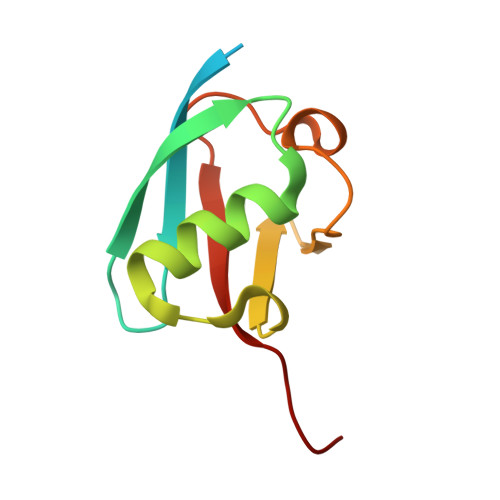
Structural insights into E1 recognition and the ubiquitin-conjugating activity of the E2 enzyme Cdc34.
Williams, K.M., Qie, S., Atkison, J.H., Salazar-Arango, S., Alan Diehl, J., Olsen, S.K.(2019) Nat Commun 10: 3296-3296
- PubMed: 31341161
- DOI: https://doi.org/10.1038/s41467-019-11061-8
- Primary Citation of Related Structures:
6NYA, 6NYD, 6NYO - PubMed Abstract:
Ubiquitin (Ub) signaling requires the sequential interactions and activities of three enzymes, E1, E2, and E3. Cdc34 is an E2 that plays a key role in regulating cell cycle progression and requires unique structural elements to function. The molecular basis by which Cdc34 engages its E1 and the structural mechanisms by which its unique C-terminal extension functions in Cdc34 activity are unknown. Here, we present crystal structures of Cdc34 alone and in complex with E1, and a Cdc34~Ub thioester mimetic that represents the product of Uba1-Cdc34 Ub transthiolation. These structures reveal conformational changes in Uba1 and Cdc34 and a unique binding mode that are required for transthiolation. The Cdc34~Ub structure reveals contacts between the Cdc34 C-terminal extension and Ub that stabilize Cdc34~Ub in a closed conformation and are critical for Ub discharge. Altogether, our structural, biochemical, and cell-based studies provide insights into the molecular mechanisms by which Cdc34 function in cells.
Organizational Affiliation:
Department of Biochemistry & Molecular Biology and Hollings Cancer Center, Medical University of South Carolina, Charleston, SC, 29425, USA.