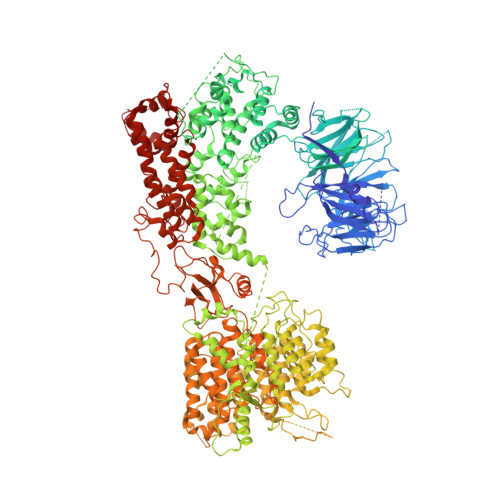
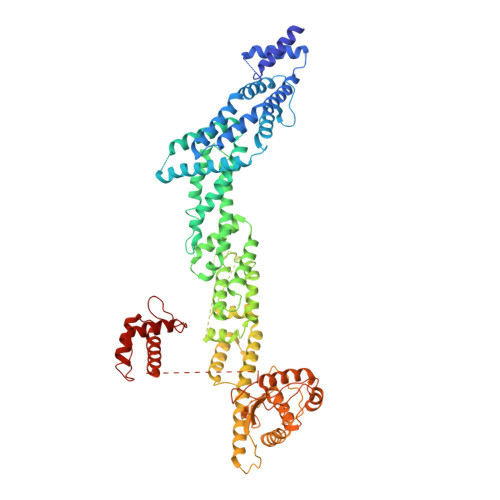
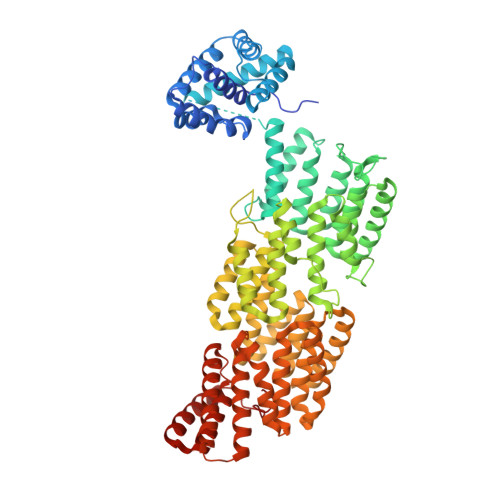
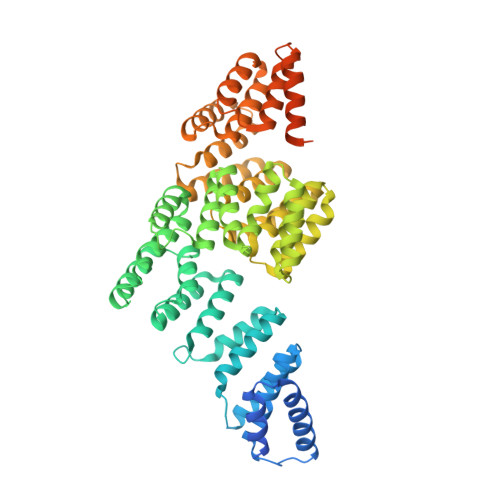
A unique binding mode of Nek2A to the APC/C allows its ubiquitination during prometaphase.
Alfieri, C., Tischer, T., Barford, D.(2020) EMBO Rep 21: e49831-e49831
- PubMed: 32307883
- DOI: https://doi.org/10.15252/embr.201949831
- Primary Citation of Related Structures:
6TLJ, 6TM5 - PubMed Abstract:
The anaphase-promoting complex (APC/C) is the key E3 ubiquitin ligase which directs mitotic progression and exit by catalysing the sequential ubiquitination of specific substrates. The activity of the APC/C in mitosis is restrained by the spindle assembly checkpoint (SAC), which coordinates chromosome segregation with the assembly of the mitotic spindle. The SAC effector is the mitotic checkpoint complex (MCC), which binds and inhibits the APC/C. It is incompletely understood how the APC/C switches substrate specificity in a cell cycle-specific manner. For instance, it is unclear how in prometaphase, when APC/C activity towards cyclin B and securin is repressed by the MCC, the kinase Nek2A is ubiquitinated. Here, we combine biochemical and structural analysis with functional studies in cells to show that Nek2A is a conformational-specific binder of the APC/C-MCC complex (APC/C MCC ) and that, in contrast to cyclin A, Nek2A can be ubiquitinated efficiently by the APC/C in conjunction with both the E2 enzymes UbcH10 and UbcH5. We propose that these special features of Nek2A allow its prometaphase-specific ubiquitination.
- MRC Laboratory of Molecular Biology, Cambridge, UK.
Organizational Affiliation: