Diverse Antibody Responses to Conserved Structural Motifs in Plasmodium falciparum Circumsporozoite Protein.
Pholcharee, T., Oyen, D., Torres, J.L., Flores-Garcia, Y., Martin, G.M., Gonzalez-Paez, G.E., Emerling, D., Volkmuth, W., Locke, E., King, C.R., Zavala, F., Ward, A.B., Wilson, I.A.(2020) J Mol Biol 432: 1048-1063
- PubMed: 31883801
- DOI: https://doi.org/10.1016/j.jmb.2019.12.029
- Primary Citation of Related Structures:
6UC5 - PubMed Abstract:
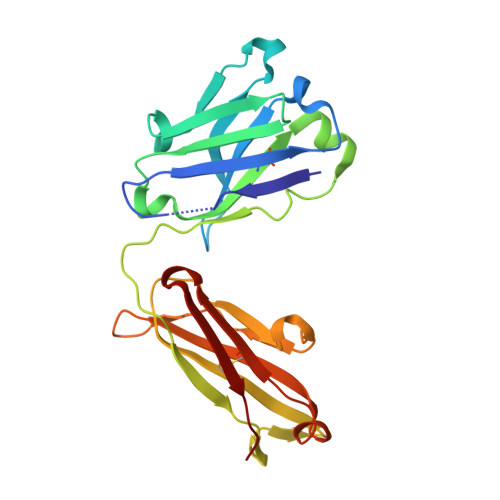
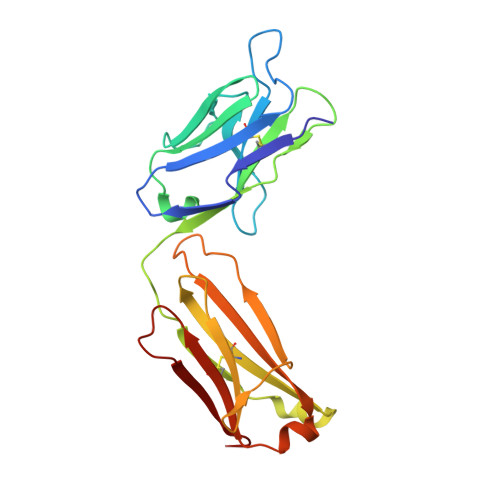
Malaria vaccine candidate RTS,S/AS01 is based on the central and C-terminal regions of the circumsporozoite protein (CSP) of P. falciparum. mAb397 was isolated from a volunteer in an RTS,S/AS01 clinical trial, and it protects mice from infection by malaria sporozoites. However, mAb397 originates from the less commonly used VH3-15 germline gene compared to the VH3-30/33 antibodies generally elicited by RTS,S to the central NANP repeat region of CSP. The crystal structure of mAb397 with an NPNA 4 peptide shows that the central NPNA forms a type I β-turn and is the main recognition motif. In most anti-NANP antibodies studied to date, a germline-encoded Trp is used to engage the Pro in NPNA β-turns, but here the Trp interacts with the first Asn. This "conserved" Trp, however, can arise from different germline genes and be located in the heavy or the light chain. Variation in the terminal ψ angles of the NPNA β-turns results in different dispositions of the subsequent NPNA and, hence, different stoichiometries and modes of antibody binding to rsCSP. Diverse protective antibodies against NANP repeats are therefore not limited to a single germline gene response or mode of binding.
Organizational Affiliation:
Department of Integrative Structural and Computational Biology, The Scripps Research Institute, La Jolla, CA, 92037, USA.